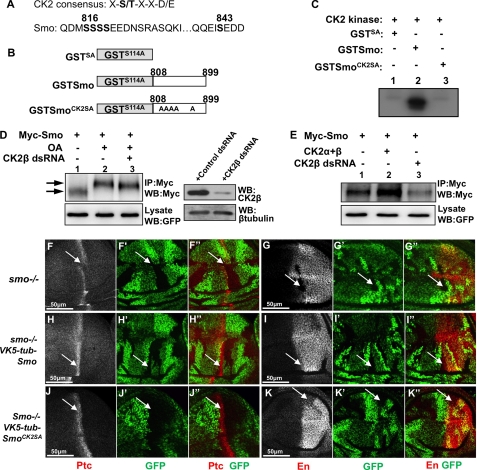
FIGURE 3.
Smo is phosphorylated by CK2. A, CK2 consensus sequence and Smo sequence indicating putative CK2 phosphorylation sites. B, schematic drawing of GST-Smo constructs that were expressed in bacteria. C, CK2 phosphorylates Smo in vitro. Shown here is an in vitro kinase assay by using the purified GST-Smo fusion proteins and the commercial CK2 kinase. D, S2 cells transfected with Myc-Smo and treated with OA or with OA and CK2β dsRNA. Cell extracts were immunoprecipitated and blotted with anti-Myc antibody to detect Smo phosphorylation indicated by its mobility shift on the SDS gel. Arrow indicates hyperphosphorylated forms of Smo, and arrowhead indicates hypophosphorylated and unphosphorylated forms. GFP served as transfection control. Special lysis buffer containing NaF and Na3VO4 was used to examine Smo phosphorylation. The efficiency of CK2β RNAi is shown in the right panel where β-tubulin served as loading control. E, Myc-Smo transfected into S2 cells either with FLAG-CK2α and FLAG-CK2β or with the treatment of CK2β dsRNA. Cell extracts were immunoprecipitated (IP) with anti-Myc and blotted (WB) with anti-Myc to examine the levels of Smo. Regular lysis buffer was used to examine Smo levels. GFP served as transfection control. F–G′, wing discs carrying smo mutant clones immunostained for Ptc (red), En (red), or GFP (green). H–I′, wing discs containing smo mutant clones and expressing VK5-Myc-Smo by tubulinα promoter immunostained to show Ptc, En, and GFP. J–K′, wing discs containing smo mutant clones and expressing VK5-Myc-SmoCK2SA by tubulinα promoter immunostained to show Ptc, En, and GFP. smo mutant clones are recognized by the lack of GFP expression.