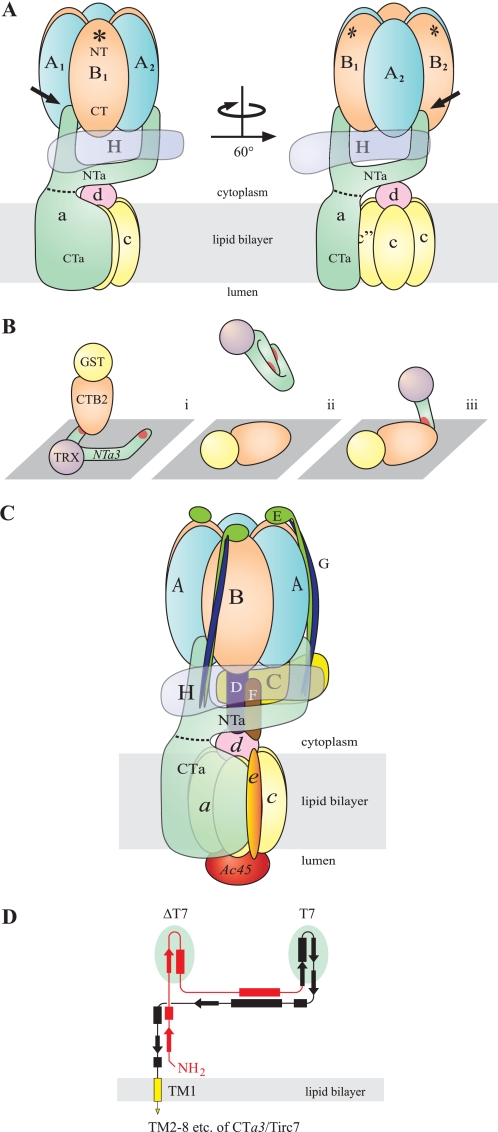
FIGURE 10.
Possible scenario for NTa interaction with the B subunit. A, for clarity, the C–G, e, and Ac45 subunits of the complex have been omitted (compare with C). Shown are the V1 sector, with catalytic head group comprising three each of alternating A and B subunits (blue and orange, respectively), and the H subunit (transparent purple). The V0 sector includes subunit a (green), with the cytoplasmic NTa and integral membrane CTa domains delimited (dashed line), the mammalian hexameric c5c″ proteolipid barrel (yellow), and subunit d (pink). Note that the B subunit is oriented with the N-terminal domain (NT) to the top (distal to the V1/V0 interface) and C-terminal domain (CT) to the bottom (see “Discussion”). Asterisks indicate the F-actin-binding site, which is localized to the NT domain in all B subunits. This diagram is patterned loosely after single particle EM reconstruction by Zhang et al. (18) and shows a bifurcated NTa with the left finger extended to the AB groove of the catalytic head group (left figure, black arrow). A 60° rotation, as indicated, shows the insertion of the right finger into the adjacent AB groove (right figure, black arrow). This takes into account interactions known to take place with both the A and B subunits. The “beam” that extends between the fingers is shown to cradle the H subunit and contact the d subunit, although this latter interaction is likely transient or manifest only when V1 and V0 are dissociated. These interactions have been demonstrated elsewhere (25, 59), but the precise assembly is speculative. Both ends of the beam, which traverses ∼120° around the rotor axis, are thought to support one each of three EG heterodimer peripheral stalks, and one end contacts the C subunit, which supports the third EG heterodimer at its distal end (see also C). Although the orientation shown here seems to be in reasonable agreement with the more detailed reconstruction images of Zhang et al. (18), the resolution of their images does not permit definitively choosing this scenario over one where the fingers of NTa are inserted into BA grooves, rather than an AB grooves, which would require only slight adjustment of the present model. In fact neither the reconstruction by Zhang et al. (18), nor similar work done by Muench et al. (24) resolves a potential “left finger” in contact with the catalytic head group in any orientation in their electron density maps. Better resolution has been obtained for T. thermophilus V-ATPase, which favors the scenario presented here (44). B, depicts an explanation for the data in Fig. 4B; panel i, the fusion protein ligand, TRX-NTa3, is splayed out, due to interaction with the hydrophobic plastic surface of the ELISA plate and is consequently accessible to the analyte, GST-CTB2, resulting in a high affinity interaction with either of the apparently equivalent binding sites (red) on the “fingers” of NTa3; panel ii,, in the reverse assay, the ligand is GST-CTB2 and the analyte is TRX-NTa3. This panel suggests that the unwieldy fingers of the intact NTa3 moiety fold on themselves while in free solution, unsupported by other subunits of the native V-ATPase. This results in sequestering its binding sites, resulting in significant reduction in binding affinity; panel iii, isolated NTa3ΔT7 or NTa3T7 domains (i.e. the isolated fingers of NTa3) are not self-sequestering and interact with immobilized CTB2 at higher affinity. C, this schematic shows the best-guess scenario of mammalian V-ATPase structure, taking into account this work and single-particle EM descriptions (18, 24, 44). It also suggests, speculatively, the co-alignment of the fingers of the a subunit with two of the three EG peripheral stalks, the third being supported by the C subunit. Recently, Lee et al. (60) have shown that the EG heterodimer forms a rare right-handed coiled-coil in T. thermophilus, also depicted here. Some subunits are depicted as semi-transparent for clarity (see also description for A). D shows a scale redrawing of the proposed secondary and tertiary structure of the NTa domain shown in Zhang et al. (18) to accommodate the present findings. It depicts the ΔT7 (red) and T7 (black) domains (with underlying green ovals indicating the two speculative fingers), which must have equivalent binding sites for the B subunit. No repetitive polypeptide sequences have been identified to suggest identical binding sites. The C-terminal end of the T7 domain continues as the CTa domain (yellow; truncated here), which includes eight predicted transmembrane α-helices (one shown) and a cytoplasmic C-terminal tail (61).