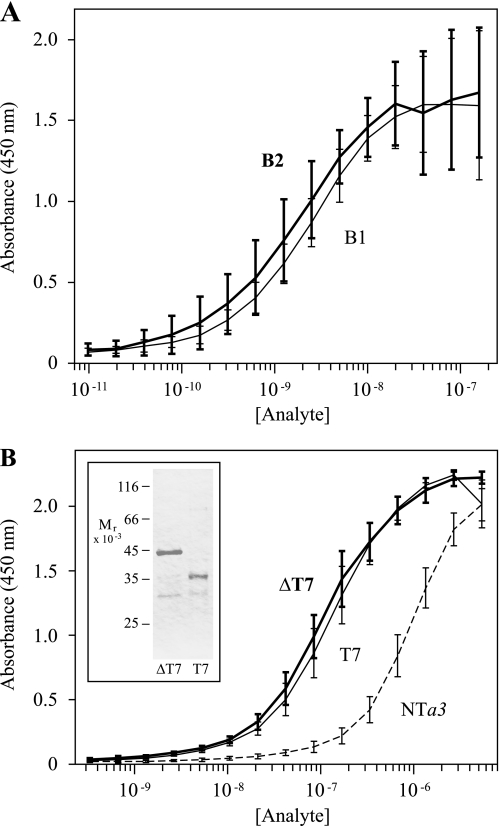
FIGURE 3.
ELISA-based binding assays of NTa3 interactions with B1 and B2 subunits. A shows ELISA saturation curves of B1 (heavy line and error bars) and B2 (light line and error bars) binding to NTa3. ELISA plates were coated with TRX-NTa3 ligand and probed with 2-fold serial dilutions of analyte (GST-B1 or GST-B2 fusion proteins, 9.8 pm to 160 nm). Binding of GST alone was negligible, as were the signals obtained without ligand or either analyte protein (data not shown). Absorbance at 450 nm was determined after staining with an anti-GST-HRP sandwich and color development with 3,3′,5,5′-tetramethylbenzidine (see “Experimental Procedures”). Absorbance at the reference wavelength of 600 nm was subtracted to normalize optical variance among wells (A600 was typically <3% of the maximum A450 signal). Each curve shows means ± S.D. bars (n = 3, in duplicate). Differences between B1 and B2 interacting with NTa3 were not significant. B shows ELISA saturation curves of NTa3ΔT7 (heavy line and error bars), NTa3T7 (light line and error bars), and NTa3(dashed line) binding to CTB2. ELISA plates were coated with GST-CTB2 ligand and probed with 2-fold serial dilutions of analyte (TRX-NTa3ΔT7, TRX-NTa3T7, or TRX-NTa3 fusion proteins, 25 pm to 400 nm). Shown also is purification of TRX-NTa3ΔT7 and TRX-NTa3T7 (SDS-PAGE, inset panel). TRX alone showed negligible binding (data not shown). Each curve shows means ± S.D. bars (n = 3, in duplicate).