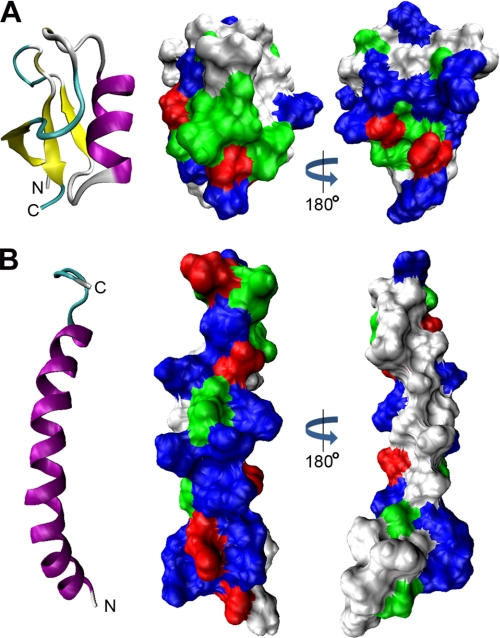
FIGURE 8.
Surface charge distribution of NaD1 and LL-37. A and B, comparison of the three-dimensional structures and surface charge distributions of NaD1 (Protein Data Bank code 1MR4) and LL-37 (code 2K6O), respectively. Ribbon diagrams show β-strands in yellow and α-helices in purple. Space-filling models show basic residues in blue, acidic residues in red, polar residues in green, and hydrophobic residues in white. A, NaD1 contains a conserved triple-stranded antiparallel β-sheet tethered to an α-helix. The molecule is hydrophilic and lacks a large hydrophobic face. B, LL-37 forms a single amphipathic α-helix.