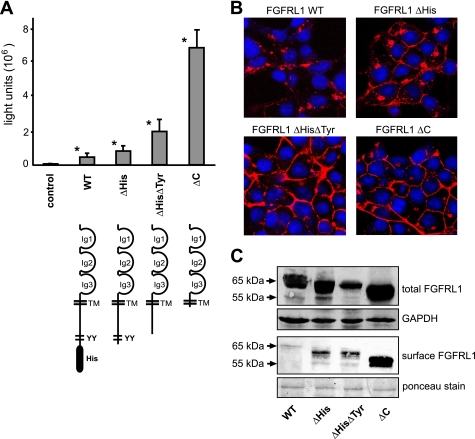
FIGURE 3.
Role of the cytoplasmic domain on the fusion process. A, CHO-K1 cells were transfected with a tetracycline-inducible luciferase expression construct (pTRE-Luc) and seeded together with HEK-TetOn cells that had been transfected with different C-terminally truncated FGFRL1 expression constructs (the corresponding proteins are schematically shown below the respective bars). The luciferase activity, measured 14 h postseeding, correlated well with the visible extent of cell-cell fusion induced by the mutated FGFRL1 constructs. The bars represent the average of three experiments. Successive truncation of cytoplasmic internalization motifs led to increased fusion, with strongest fusion observed for the completely truncated construct FGFRL1ΔC. Note that the wild-type, full-length protein also induces considerable fusion when compared with nontransfected control cells. Asterisks next to bars indicate statistically significant results (p < 0.05) compared with the control group. B, confocal images of immunofluorescent stainings of the C-terminally truncated FGFRL1 proteins. Note that the wild-type protein resided primarily in intracellular compartments, while the truncated forms accumulated in the plasma membrane. C, surface biotinylation of mutated FGFRL1 proteins. HEK293 cells stably expressing the indicated FGFRL1 proteins were subjected to surface biotinylation, followed by isolation with neutravidin agarose and detection of biotinylated FGFRL1 by Western blotting. The upper panel shows total FGFRL1 in the respective cells, with GAPDH as a loading control. The lower panel shows biotinylated FGFRL1 from the cell surface. Ponceau staining of a major membrane protein of ∼85 kDa served as a loading control for the neutravidin purification.