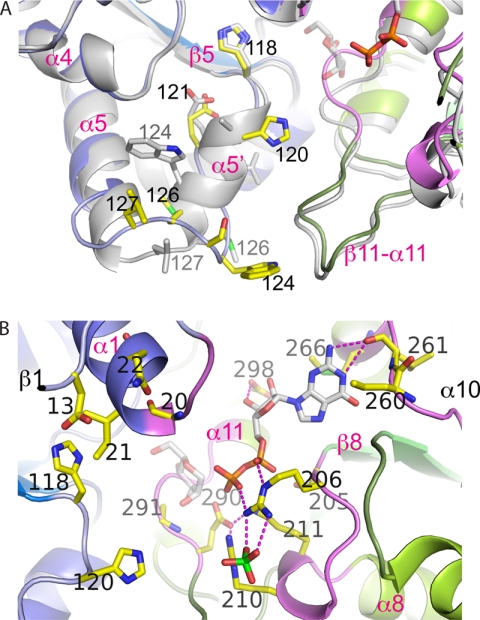
FIGURE 3.
Structural flexibility in the β5-α5 loop and overview of the active site of PimB′. A, comparison of conformation between the triclinic (green/blue) and orthorhombic crystal (gray) forms of PimB′. Helix α5′ is only present in the orthorhombic form. B, close-up view of the active site cleft of triclinic PimB′ in the open configuration with ribbons in blue and green indicating the N- and C-terminal domains, respectively. Loop regions involved in nucleotide binding are colored in magenta. Residues in contact with nucleotide or that were probed by point mutagenesis are shown in yellow sticks. The nucleotide shown reflects the “PimA-like” conformation of the pyrophosphate. The mannose moiety is shown in transparent sticks, modeled according to the conformation seen in OtsA.