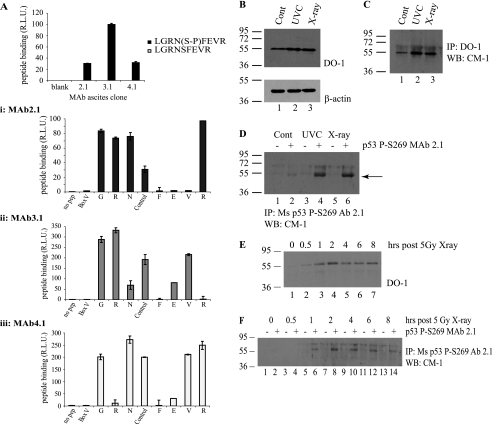
FIGURE 8.
In vivo phosphorylation of p53 at serine 269; detection using phosphospecific antibodies. A, phosphospecific antibody generation to serine 269. Monoclonal antibodies (mAb 2.1, 3.1, and 4.1) were generated toward phospho-Ser269 peptides, and peptide ELISA was used to demonstrate the phosphospecificity of the antibodies. A, i–iii, serine 269 mAb (i, mAb 2.1; ii, mAb 3.1; iii, mAb 4.1) epitopes were mapped via ELISA using an alanine scan mutation of GRNpSFEVR phosphopeptide. B–D, Ser269-phosphospecific mAb 2.1 can immunoprecipitate p53 from MCF7 lysates following DNA damage. MCF7 cells were exposed to 5 grays of x-ray or 10 J cm2 UV irradiation, and induction of p53 protein levels was analyzed 6 h later by immunoblotting with DO-1 (B, showing 10 μg of cellular lysate probed with DO-1 (top) and β-actin (bottom) as a loading control). Total p53 protein and Ser269-phosphorylated p53 were isolated from these lysates by immunoprecipitation with DO-1 (C) or mAb 2.1 (D), respectively, and an increase in Ser269 phosphorylation can be detected (D, lanes 4 and 6). Protein G beads were used as a control for both DO-1 and mAb 2.1 immunoprecipitation and are shown in D in lanes marked with minus signs. This increase in phosphorylation with phosphospecific antibody after irradiation is consistent with the pool of DO-12 non-reactive p53 protein in the nucleus of cells after UV radiation (Fig. 7, C versus D). E–F, time scale of p53 Ser269 phosphorylation following DNA damage. MCF-7 cells were irradiated with 5 grays of x-ray radiation and harvested at the indicated time points. Increases in total p53 (E) or phospho-Ser269 p53 (F) protein levels were determined by immunoblotting cellular lysates with DO-1 (E) or immunoprecipitating phospho-Ser269 p53 from cellular lysates using protein G beads without (−) or with mAb 2.1 (+), followed by immunoblotting with polyclonal CM-1 (F). R.L.U., relative light units; IP, immunoprecipitation; IB, immunoblot.