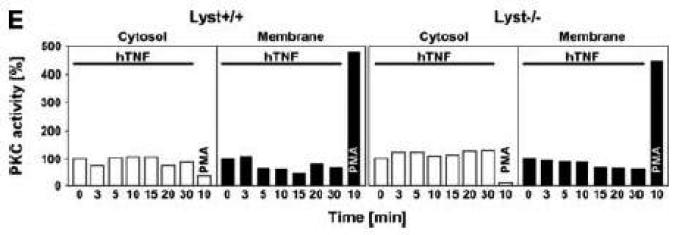
Figure 2.
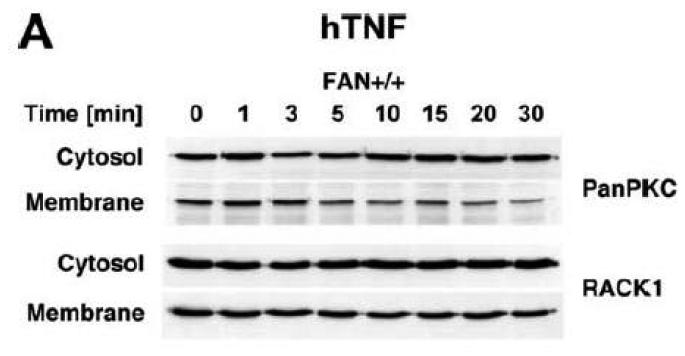
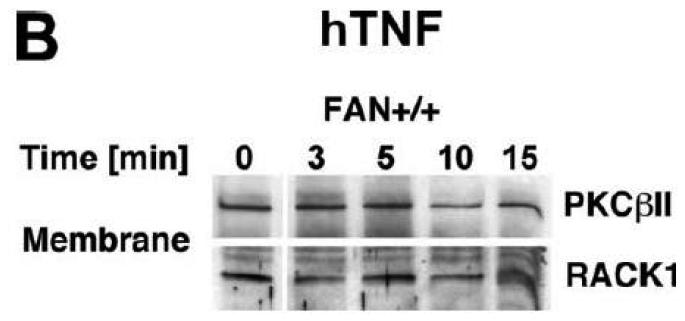
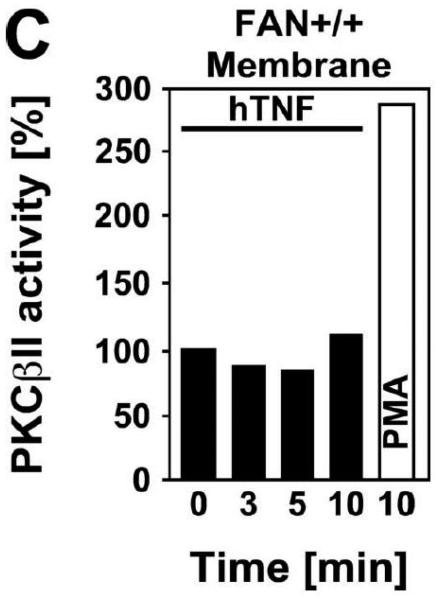
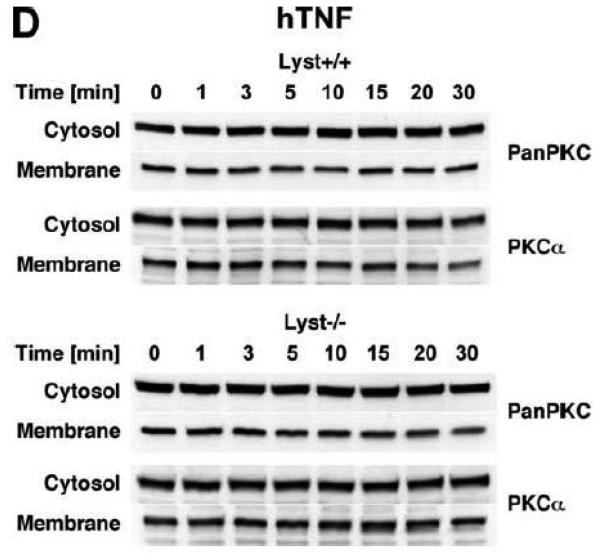
TNF does not induce membrane translocation or activation of PKC isotypes in fibroblasts. (A) EF from mice harboring a functional FAN protein (FAN+/+) were treated with 100 ng/ml hTNF for the indicated times. Cytosolic and membrane fractions were analyzed for the presence of cPKC isotypes (using an antibody simultaneously recognizing all cPKC isotypes; “panPKC”) or for presence of RACK1. Detection of β-actin was used as a loading control (data not shown). (B) FAN+/+ EF were treated with 50 ng/ml hTNF for the indicated times and PKCβII was immunoprecipitated from the membrane fraction. Subsequently, RACK1 coimmunoprecipitating with PKCβII and (for control of the immunoprecipitation reaction) PKCβII itself were detected by Western blot. (C) PKCβII activity was measured in immunoprecipitates from FAN+/+ EF that were generated as in panel (B). PKCβII activity measured in immunoprecipitates from FAN+/+ EF after treatment with 0.1 μM PMA for 10 min is shown for comparison. One out of two experiments with similar results is shown. (D) Fibroblasts from wildtype (Lyst+/+) and from Lyst-deficient (Lyst−/−) mice were treated and analyzed for membrane translocation of cPKC and PKCα by Western blot as in panel (A). Detection of β-actin was used as a loading control (data not shown). For detection of PKCα, membranes probed with panPKC were reprobed with an antibody specific for PKCα. Prior to reprobing, any residual peroxidase activity was eliminated by incubating the membranes in 15 % v/v H202. Furthermore, the utilized pan PKC and PKCα antibodies were generated in different species, thus ensuring the specificity of detection. (E), Cytosolic and membrane fractions from Lyst+/+ and Lyst−/− fibroblasts were assayed for PKC activity after stimulation with 50 ng/ml hTNF for the indicated times. Activity is shown relative to unstimulated cells. Treatment with 0.1 μM PMA for 10 min is shown for comparison.