Figure 3. AVV-nNOS injection into the HN mimics antidromic latency alterations induced by XIIth nerve crushing.
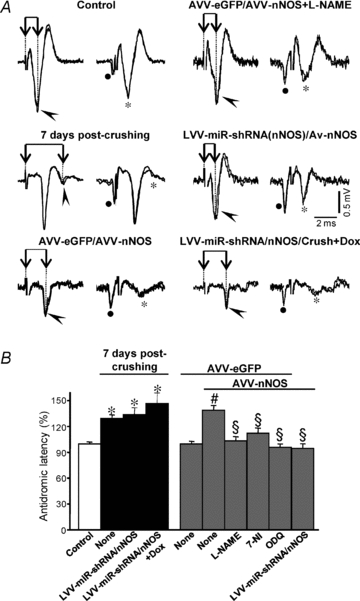
A, representative antidromic activations (arrowheads) and collisions (asterisks) in motoneurons recorded at the indicated conditions. Activation latency was measured as the time difference between XIIth nerve stimulation artifact (dotted line) and the negative peak of the antidromic spike (connected arrows). B, average antidromic activation latency (in percent) measured at the indicated conditions. Control condition was taken as 100%. The number of analysed HMNs per condition was as follows: control, n = 112; 7 days post-crushing+None, n = 55; 7 days post-crushing+LVV-miR-shRNA/nNOS, n = 15; 7 days post-crushing+LVV-miR-shRNA/nNOS+Dox, n = 15; AVV-eGFP, n = 30; AVV-eGFP/AVV-nNOS, n = 41; AVV-eGFP/AVV-nNOS+l-NAME, n = 33; AVV-eGFP/AVV-nNOS+7-NI, n = 38; AVV-eGFP/AVV-nNOS+ODQ, n = 35; AVV-eGFP/AVV-nNOS+LVV-miR-shRNA/nNOS, n = 25. Significant differences (P < 0.05; one-way ANOVA; post hoc Tukey's test) relative to control (*), AVV-eGFP- (#) or AVV-eGFP/AVV-nNOS-injected (§) groups. AVV-nNOS-induced latency alterations were fully prevented by systemic administration of 7-NI or ODQ and intranuclear injection of LVV-miR-shRNA/nNOS. However, lentivirus was not protective against crushing-provoked changes in latency.
