Abstract
Purpose
To determine if alemtuzumab consolidation improves response rate and progression-free survival (PFS) after induction chemoimmunotherapy in previously untreated symptomatic patients with chronic lymphocytic leukemia.
Patients and Methods
Patients (n = 102) received fludarabine 25 mg/m2 intravenously days 1 to 5 and rituximab 50 mg/m2 day 1, 325 mg/m2 day 3, and 375 mg/m2 day 5 of cycle 1 and then 375 mg/m2 day 1 of cycles 2 to 6; fludarabine plus rituximab (FR) administration was repeated every 28 days for six cycles. Three months after completion of FR, patients with stable disease or better response received subcutaneous alemtuzumab 3 mg day 1, 10 mg day 3, and 30 mg day 5 and then 30 mg three times per week for 5 weeks.
Results
Overall response (OR), complete response (CR), and partial response (PR) rates were 90%, 29%, and 61% after FR, respectively; 15% of patients were minimal residual disease (MRD) negative. Of 102 patients, 58 received alemtuzumab; 28 (61%) of 46 patients achieving PR after FR attained CR after alemtuzumab. By intent to treat (n = 102), OR and CR rates were 90% and 57% after alemtuzumab, respectively; 42% of patients became MRD negative. With median follow-up of 36 months, median PFS was 36 months, 2-year PFS was 72%, and 2-year OS was 86%. In patients achieving CR after FR, alemtuzumab was associated with five deaths resulting from infection (viral and Listeria meningitis and Legionella, cytomegalovirus, and Pneumocystis pneumonias), which occurred up to 7 months after last therapy. The study was amended to exclude CR patients from receiving alemtuzumab.
Conclusion
Alemtuzumab consolidation improved CR and MRD-negative rates after FR induction but caused serious infections in patients who had already achieved CR after induction and did not improve 2-year PFS or survival.
INTRODUCTION
The development of rituximab1,2 and chemoimmunotherapy regimens, such as fludarabine plus rituximab (FR)3,4 and fludarabine, cyclophosphamide, and rituximab,5–7 has improved complete response (CR) and overall response (OR) rates and progression-free survival (PFS) in previously untreated patients with chronic lymphocytic leukemia (CLL). Despite these advances, patients with CLL invariably relapse and typically become resistant to therapy. An important clinical question concerns the value of eliminating minimal residual disease (MRD). Studies generally have demonstrated that patients attaining partial response (PR) have shorter PFS than those attaining CR.5,6,8,9 The monoclonal anti-CD52 antibody alemtuzumab (Campath-1H; Genzyme, Cambridge, MA) is approved for both previously untreated and relapsed CLL.10,11 Alemtuzumab is particularly effective against peripheral blood and bone marrow disease, and pilot studies demonstrated that alemtuzumab effectively eradicates disease remaining after different induction therapies.12,13 These findings prompted sequential studies in which induction treatment with fludarabine or fludarabine-based combinations was administered followed by consolidation alemtuzumab to determine if such regimens improved outcome.14–16
In the CALGB (Cancer and Leukemia Group B) 19901 study, alemtuzumab was administered intravenously (IV) or subcutaneously (SC) three times per week for 6 weeks as consolidation after four cycles of fludarabine induction therapy.17 Cytomegalovirus (CMV) reactivation occurred in nine of 59 patients, but infectious toxicity was otherwise acceptable. Infusion toxicity associated with IV alemtuzumab was markedly reduced by SC administration, and both IV and SC alemtuzumab improved the CR rate—but not PFS—compared with a previous fludarabine-based phase III study by our group. An Italian study, in which SC alemtuzumab 10 mg was administered three times per week for 6 weeks in patients who had responded to fludarabine-based induction therapy, demonstrated that SC alemtuzumab effectively eliminated MRD with acceptable toxicity, but PFS data were not included, because most patients underwent autologous stem-cell transplantation.18 The German CLL4B study, which randomly assigned patients achieving PR or CR after fludarabine or fludarabine plus cyclophosphamide to observation or IV alemtuzumab 30 mg three times per week for 12 weeks, demonstrated improved PFS, but significant infectious morbidity was also noted.14,15 However, none of these studies included rituximab administration in the induction regimen. Thus, the clinical benefit of alemtuzumab consolidation after chemoimmunotherapy has remained unclear. The CALGB 10101 study was initiated with the rationale that a 3-month waiting period before initiating SC alemtuzumab consolidation would diminish toxicity and improve clinical outcome after FR induction therapy. The results demonstrate significant toxicity with this regimen and no clear benefit over FR alone, compared with our previous CALGB 9712 study.3,4
PATIENTS AND METHODS
Patients
Patients were enrolled onto this National Cancer Institute (NCI)–sponsored clinical study after approval by the institutional review boards of participating CALGB centers. Eligible patients provided written informed consent, had CLL by NCI 96 criteria,19 had high-risk Rai stage III/IV disease or active intermediate-risk Rai stage I/II disease, were age 18 years or older, had received no prior therapy including steroids for autoimmune complications, were not receiving chronic corticosteroids, had Eastern Cooperative Oncology Group performance status 0 to 2, and met the following criteria: creatinine less than 1.5 times the institutional upper limit of normal (ULN), bilirubin less than two times the ULN, transaminases two times or less than the ULN, and a negative direct antibody test. Pregnant women were excluded.
Treatment Plan
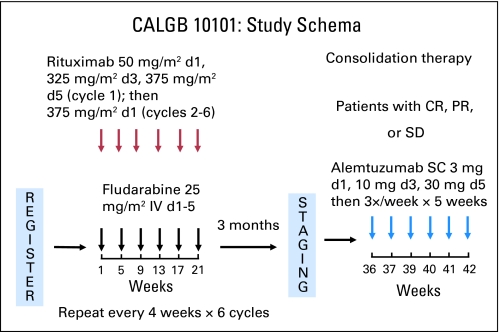
Patients received fludarabine 25 mg/m2 IV on days 1 to 5 of each cycle and rituximab 50 mg/m2 IV on day 1, 325 mg/m2 on day 3, and 375 mg/m2 on day 5 of cycle 1 and then 375 mg/m2 on day 1 of cycles 2 to 6; FR was repeated every 28 days for six cycles (Fig 1). Allopurinol 300 mg was administered on days 1 to 14 of cycle 1, and prophylaxis for Pneumocystis pneumonia (PCP) and Varicella zoster virus occurred at investigator discretion during FR administration. Three months after completion of the final induction cycle, patients with stable disease (SD) or responsive disease received SC alemtuzumab 3 mg on day 1, 10 mg on day 3, and 30 mg on day 5 and then 30 mg three times per week for 5 weeks (total dose, 493 mg). PCP and Varicella zoster virus prophylaxis were required throughout and for 6 months after completion of alemtuzumab. CMV monitoring was performed weekly during alemtuzumab administration and every 2 weeks for 2 additional months. Growth factors were not encouraged but were allowed according to American Society of Clinical Oncology guidelines.
Fig 1.
Study schema for the CALGB (Cancer and Leukemia Group B) 10101 study. Patients received fludarabine 25 mg/m2 intravenously (IV) on days (d) 1 to 5 and rituximab 50 mg/m2 IV on d 1, 325 mg/m2 on d 3, and 375 mg/m2 on d 5 of cycle 1 and then 375 mg/m2 on d 1 of cycles 2 to 6; this was repeated every 28 d for 6 cycles. Three months after completing the final induction cycle, patients achieving stable disease (SD), partial response (PR), or complete response (CR) received alemtuzumab 3 mg subcutaneously (SC) on d 1, 10 mg on d 3, and 30 mg on d 5 and then 30 mg three times per week for 5 weeks (total dose, 493 mg).
Assessment of Toxicity, Response, and MRD
NCI Common Toxicity Criteria Version 2.0 was used to evaluate toxicity. In toxicity monitoring of alemtuzumab, unacceptable toxicity was defined as injection-related toxicity resulting in patient removal from protocol treatment, grade 4 to 5 infection, grade 3 to 5 opportunistic infection, and grade 3 to 5 nonhematologic toxicity during or after alemtuzumab therapy. Patients were assessed for clinical response using NCI 96 criteria 3 months after FR therapy ended and again 2 months after completion of alemtuzumab.19 Given that many patients had significant cytoreduction after FR therapy, we also assessed improvement in response (SD to PR and PR to CR) in patients receiving alemtuzumab consolidation. Determination of MRD status was performed by flow cytometry according to local institutional standards.
Statistical Analysis
The primary objective of this nonrandomized phase II trial was to assess the toxicity and response rate of FR induction therapy followed by alemtuzumab consolidation therapy. The planned sample size was 100 patients. Toxicity was monitored during alemtuzumab consolidation using a customized seven-stage design, with seven stopping points at which adverse event data were reviewed for excessive toxicity on the basis of predefined criteria. This design had a 0.90 probability of rejection of the null hypotheses of a toxicity rate of 35%, if the true toxicity rate were 20%. Overall survival (OS) was calculated as the time from the date of study registration to death as a result of any cause or last contact for living patients. PFS was defined as the time from the date of study registration to the first occurrence of either disease progression or death as a result of any cause or last contact for living patients. Survival probabilities were calculated using the Kaplan-Meier method.20 A two-sided log-rank test was used to test for differences in survival times between subsets of patients.21 Statistical significance was defined as P < .05. Follow-up time was calculated using the potential follow-up method.22 Follow-up (median, 36 months; range, 8 to 55 months) for surviving patients was current as of October 19, 2009.
Patient registration, data collection, and all analyses were performed by the CALGB Statistical Center (Durham, NC). Data quality was ensured by review of the data by center staff and the study chairperson in adherence with standard CALGB policies. The CALGB Audit Committee visits all participating institutions at least once every 3 years to verify compliance with federal regulations and protocol requirements for CALGB studies, including those pertaining to eligibility, treatment, response, and follow-up.
RESULTS
Patient Characteristics
From January 2005 to December 2006, 103 patients were enrolled. One patient was determined ineligible because of concurrent Richter's transformation, withdrawn from protocol treatment after cycle 1 of FR, and excluded from the efficacy analysis. Table 1 describes the 102 patients in the primary efficacy analysis. Median age was 61 years (range, 23 to 82 years), and 75 patients (74%) were male. Thirty patients (30%) had high-risk Rai stage III/IV disease, 71 patients (69%) had intermediate-risk Rai stage I/II disease, and one patient (1%) had symptomatic Rai stage 0 disease.
Table 1.
Patient Characteristics and Delivery of Therapy (n = 102)
| Characteristic | Patients |
|
|---|---|---|
| No. | % | |
| Age, years | ||
| Median | 61 | |
| Range | 23-82 | |
| Male | 75 | 74 |
| Rai stage | ||
| 0 | 1 | 1 |
| I-II | 71 | 69 |
| III-IV | 30 | 30 |
| FR induction | ||
| No. of cycles completed | ||
| ≥ 3 | 95 | 93 |
| 6 | 79 | 77 |
| Alemtuzumab consolidation (n = 58) | ||
| No. of weeks completed | ||
| ≥ 3 | 53 | 91 |
| 6 | 42 | 72 |
Abbreviation: FR, fludarabine plus rituximab.
Delivery of Therapy
FR induction therapy was generally well tolerated (Table 1). Ninety-three percent of patients completed at least three cycles of therapy, and 77% of patients completed all six planned cycles. Fifty-eight patients received alemtuzumab consolidation (Table 1). Forty-four patients did not receive alemtuzumab for the following reasons: CR (17 patients), PR with no marrow disease (one patient), progression (five patients), failure to respond to FR (three patients), infection (four patients), patient refusal (four patients), rash/hives (two patients), unrelated stroke (one patient), unrelated liver abnormalities (one patient), and unknown (two patients). Fifty-three patients received at least 3 weeks of alemtuzumab, and 42 patients (72%) completed all 6 weeks of therapy. The median delivered dose was 483 mg, and 29 of 58 patients received the full planned dose of 493 mg.
Toxicity During FR Induction
Of 102 patients, 25% and 37% developed grades 3 and 4 hematologic toxicity, respectively; 22% and 32% developed grades 3 and 4 neutropenia, respectively (Table 2). Grade 3 to 4 anemia and thrombocytopenia were noted in 7% and 12% of patients, respectively, and one patient experienced grade 3 autoimmune hemolytic anemia (AIHA). Thirty-four percent and 1% of patients experienced grades 3 and 4 nonhematologic toxicity, respectively, including 15% with febrile neutropenia and 8% with infections. The most common nonhematologic toxicities were fatigue (80%), nausea (54%), pain (51%), rash (30%), transaminitis (25%), hyperglycemia (25%), fever (24%), and vomiting (24%); however, these toxicities were overwhelmingly of grades 1 to 2 in severity (Appendix Table A1, online only).
Table 2.
Toxicity (n = 102)
| Toxicity | Grade |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
2 |
3 |
4 |
5 |
||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | |
| FR induction | ||||||||||
| Maximum hematologic | 14 | 14 | 14 | 14 | 26 | 25 | 38 | 37 | — | |
| Neutropenia | 11 | 11 | 11 | 11 | 22 | 22 | 33 | 32 | — | |
| Anemia | 43 | 42 | 21 | 21 | 6 | 6 | 1 | 1 | — | |
| Thrombocytopenia | 43 | 42 | 16 | 16 | 11 | 11 | 1 | 1 | — | |
| Maximum nonhematologic | 28 | 27 | 37 | 36 | 35 | 34 | 1 | 1 | — | |
| Febrile neutropenia | 0 | 0 | 0 | 0 | 15 | 15 | 0 | 0 | — | |
| Infection | 0 | 0 | 8 | 8 | 7 | 7 | 1 | 1 | — | |
| Alemtuzumab consolidation (n = 58) | ||||||||||
| Maximum hematologic | 7 | 12 | 9 | 16 | 14 | 24 | 23 | 40 | — | |
| Neutropenia | 11 | 19 | 4 | 7 | 15* | 26* | 10 | 17 | — | |
| Anemia | 23 | 40 | 11 | 19 | 8 | 14 | 2 | 3 | — | |
| Thrombocytopenia | 19 | 33 | 9 | 16 | 6 | 10 | 5 | 9 | — | |
| Maximum nonhematologic | 7 | 12 | 17 | 29 | 23 | 40 | 0† | 0† | 7 | 12 |
| Febrile neutropenia | 0 | 0 | 0 | 0 | 10 | 17 | 1 | 2 | — | |
| Infection | 0 | 0 | 5 | 9 | 10 | 17 | 1 | 2 | 5 | 9 |
| ARDS | 0 | 0 | 0 | 0 | — | — | 1 | 2 | ||
| Transfusion GVHD | 0 | 0 | 0 | 0 | — | — | 1 | 2 | ||
Fifteen patients had a maximum toxicity of grade 3 for neutropenia. In 14 of these patients, grade 3 was also the maximum grade for all types of hematologic toxicities. One patient had grade 3 neutropenia but had another hematologic toxicity of grade 4.
One patient had maximum toxicities of grade 4 for febrile neutropenia and infection but had an overall maximum nonhematologic toxicity of grade 5.
Abbreviations: FR, fludarabine plus rituximab; ARDS, adult respiratory distress syndrome; GVHD, graft-versus-host disease.
Severe Infectious Toxicity After Alemtuzumab Consolidation
The initial patient participating in this study died as a result of meningitis 7 months after completion of alemtuzumab therapy. At the time, the treating physician did not attribute this death to study treatment. In November 2006, the second and third deaths not related to disease progression were noted; all three deaths occurred in patients who had received alemtuzumab after achieving CR to induction. Analysis of all patients who had received alemtuzumab at that time indicated that seven (44%) of 16 patients who had achieved CR (n = 12) or had bone marrow CR but inadequate counts (n = 4) after induction had experienced unacceptable infectious or nonhematologic toxicity, as defined by the study, compared with two (10%) of 20 patients who had achieved PR after induction. In December 2006, sites with patients achieving CR after FR induction were instructed not to administer any additional alemtuzumab to these patients, and the study was amended to restrict alemtuzumab therapy to patients achieving SD or PR after induction.
Five deaths resulting from infections eventually occurred in patients who had received alemtuzumab after achieving CR to induction therapy (Table 2). In each case, CLL was in remission at time of death. One patient died from Listeria meningitis during alemtuzumab consolidation. A second patient developed CMV reactivation and glomerulonephropathy during alemtuzumab therapy, resulting in discontinuation of trimethoprim/sulfamethoxazole, and died as a result of PCP and adult respiratory distress syndrome 4.5 months after last therapy. A third patient developed Legionella pneumonia, complicated by pancytopenia and acute renal failure, 1 month after completing alemtuzumab and died as a result of adult respiratory distress syndrome and multiorgan failure 5 months later. A fourth patient who had received only four doses of alemtuzumab died as a result of sepsis 5 months after last therapy; no organism was identified. A fifth patient died as a result of viral meningitis 7 months after alemtuzumab. In addition, two patients who had achieved PR after induction and received alemtuzumab died as a result of causes unrelated to progressive CLL (Table 2). The first patient died as a result of fulminant Epstein-Barr virus viremia and hepatitis 4 weeks after alemtuzumab, and the second patient died as a result of transfusion-associated graft-versus-host disease (TA-GVHD) 8 months after alemtuzumab after receiving nonirradiated blood products after surgery.
Other Toxicity During and After Alemtuzumab Consolidation
Of 58 patients, 24% and 40% developed grades 3 and 4 hematologic toxicity, respectively; 26% and 17% developed grades 3 and 4 neutropenia, respectively (Table 2). Grade 3 to 4 anemia and thrombocytopenia were noted in 17% and 19% of patients, respectively, and three patients experienced grade 3 AIHA. Forty percent and 0% of patients experienced grades 3 and 4 nonhematologic toxicity, respectively, including 19% with febrile neutropenia and 19% with infections. As described in Severe Infectious Toxicity After Alemtuzumab Consolidation, there were seven grade 5 events. The most common nonhematologic toxicities were fatigue (72%), rash (52%), pain (43%), fever (29%), hyperglycemia (29%), nausea (28%), and pruritus (28%), which were generally of grades 1 to 2 in severity (Appendix Table A1).
Response to Therapy
One hundred two patients were evaluable for response (Table 3). OR, CR, and PR rates after FR induction were 90%, 29%, and 61%, respectively, and 15% of patients were MRD negative by flow cytometry. Of the 58 patients who received alemtuzumab, OR, CR, and PR rates were 91%, 66%, and 26%, respectively, with 50% of patients MRD negative after alemtuzumab. Twenty-eight (61%) of 46 patients achieving PR after FR who received alemtuzumab attained CR (Table 4). Of 12 patients achieving CR after FR who received alemtuzumab, five were MRD negative before consolidation, and three of the remaining seven patients converted to MRD-negative status afterward. By intent to treat for all patients enrolled (N = 103), OR, CR, and MRD negativity were attained by 90%, 57%, and 42% of patients, respectively.
Table 3.
Response to Therapy (n = 102)
| Response | After FR (%) | End of Therapy (%) |
|
|---|---|---|---|
| Alemtuzumab (n = 58) | All Patients | ||
| CR | 29 | 66 | 57 |
| PR | 61 | 26 | 33 |
| OR | 90 | 91 | 90 |
| MRD negative* | 15 | 50 | 42 |
Abbreviations: FR, fludarabine plus rituximab; CR, complete response; PR, partial response; OR, overall response; MRD, minimal residual disease.
MRD status was assessed by flow cytometry on bone marrow samples at local Cancer and Leukemia Group B institutions according to the standards of the individual institution. Because MRD assessment by flow cytometry was not an objective of this study, MRD-negative patients are expressed as a percentage of all patients, regardless of clinical response or whether bone marrow aspiration was performed.
Table 4.
Improvement of Response With Alemtuzumab Consolidation (n = 58)
| Response After FR Induction | Response After Alemtuzumab Consolidation |
|||
|---|---|---|---|---|
| CR |
MRD Negative |
|||
| No. | % | No. | % | |
| PR (n = 46) | 28 | 61 | 23 | 50 |
| CR, MRD negative (n = 5) | 5 | 100 | 5 | 100 |
| CR, MRD positive (n = 7) | 7 | 100 | 3 | 43 |
Abbreviations: FR, fludarabine plus rituximab; CR, complete response; MRD, minimal residual disease; PR, partial response.
Survival Analysis
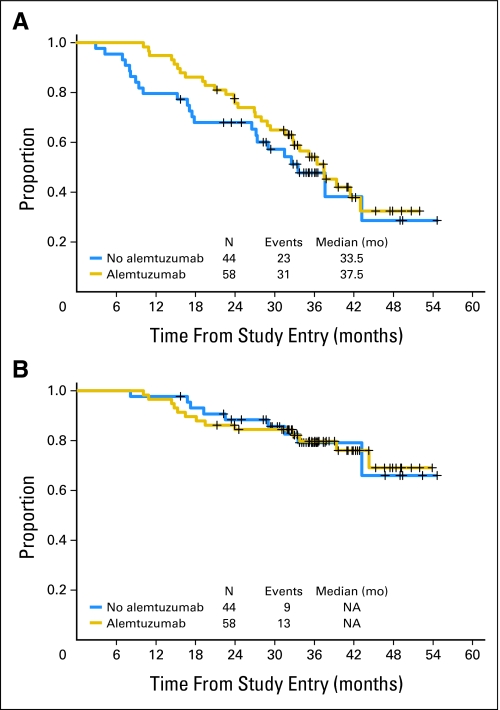
With a median follow-up of 36 months, median PFS was 36 months, with PFS of 72% and OS of 86% at 2 years. Two-year PFS (76% v 68%; P = .35) and OS (84% v 88%; P = 1.0) were similar for patients who did and did not receive alemtuzumab (Fig 2). Similarly, there were no differences in PFS or OS among the 30 patients achieving CR after FR induction, regardless of whether they received alemtuzumab (data not shown).
Fig 2.
(A) Progression-free survival and (B) overall survival in patients who received (n = 58; gold line) and did not receive alemtuzumab (n = 44; blue line), measured from study entry. NA, not achieved.
DISCUSSION
These results confirm the feasibility and safety of concurrent FR-based chemoimmunotherapy for previously untreated CLL, as reported by CALGB.3,4 Unfortunately, this study also demonstrated that the addition of SC alemtuzumab consolidation beginning 3 months after completion of induction FR resulted in unacceptable, severe infections, primarily in patients who had achieved CR after induction. Therefore, we modified eligibility criteria for consolidation treatment and restricted alemtuzumab therapy to patients who had achieved SD or PR after induction FR. Although alemtuzumab toxicity was diminished by this amendment, and improvements in CR and MRD rates were observed among patients receiving alemtuzumab, consolidation therapy failed to improve PFS or OS, compared with historical results with FR alone.4 Thus, our data do not support additional pursuit of consolidation alemtuzumab as administered in this study.
Although unexpected hematologic toxicity was not observed, four patients experienced AIHA, three of whom underwent alemtuzumab consolidation. It is unclear whether AIHA was the result of disease progression or fludarabine and whether alemtuzumab influenced the development of AIHA. Although we initiated alemtuzumab consolidation 3 months after completion of FR induction, we observed problems with infectious morbidity similar to those in previous studies, in which alemtuzumab consolidation was initiated 1 month after combination chemotherapy14,15 or chemoimmunotherapy.16 These studies were terminated or not explored further because of poor tolerability and increased toxicity. Whereas CMV reactivation was the primary infection in prior studies of alemtuzumab consolidation,12,14 we observed a broad array of grade 5 infections, including sepsis, Legionella pneumonia, PCP, Listeria and viral meningitis, and Epstein-Barr virus viremia, which occurred up to 7 months after last alemtuzumab therapy. Five of these six deaths occurred in patients who had achieved CR after FR induction, and the overall rate of unacceptable grade 3 to 5 infectious complications was similarly higher in patients achieving CR than in patients achieving SD or PR. These infections occurred during and after completion of therapy, suggesting long-lasting immunosuppression with this combined antibody-based approach. The potentially preventable deaths resulting from PCP and TA-GVHD highlight the importance of multicenter studies in defining the toxicity of new clinical treatments that may extend immunosuppression. The patient with TA-GVHD required emergent blood transfusions perioperatively for an unexpectedly complicated hernia repair. Educating both health care providers and patients about the risk of TA-GVHD is therefore essential in preventing such occurrences.
There are several potential explanations for the increased infectious toxicity and morbidity after alemtuzumab consolidation in this study. Patients achieving CR likely have minimal tumor targets for CD52-targeted alemtuzumab, resulting in increased off-target T-cell depletion and immunosuppression. Prior studies of IV alemtuzumab consolidation included few patients achieving CR after induction therapy, possibly contributing to the absence of severe infections in these studies; only one of 11 patients who received alemtuzumab in the German CLL4B study had achieved CR, and only three of 41 patients in a single-institution study of alemtuzumab consolidation had achieved CR.12,14,15 CALGB 10101 demonstrated that patients achieving CR after induction therapy were particularly vulnerable to alemtuzumab consolidation. The patients achieving CR who died after alemtuzumab were MRD positive after induction; therefore, precluding only MRD-negative patients from receiving alemtuzumab would not be sufficient to ensure patient safety.
The sequential administration of an induction regimen containing cytotoxic chemotherapy and rituximab followed by alemtuzumab may also have contributed to the observed toxicity. Although the addition of rituximab to cytotoxic chemotherapy has generally been well tolerated in CLL and lymphoma, the addition of rituximab to cyclophosphamide, doxorubicin, vincristine, and prednisone in HIV-positive patients with CD4+ T-cell counts less than 200 was associated with significant infectious morbidity and no overall benefit.23 Similarly, although most studies of alemtuzumab consolidation have involved administration of fludarabine or fludarabine plus cyclophosphamide without rituximab as induction therapy, one phase II study involved administration of four cycles of FR followed 5 weeks later by IV alemtuzumab three times per week for 4 weeks among 34 previously untreated patients with CLL.16 Only five patients achieved CR after FR. Alemtuzumab was poorly tolerated, and two patients died as a result of treatment-related toxicity. Median PFS (42 months) was similar to that in our study. Thus, the sequential administration of FR induction and alemtuzumab consolidation may produce extended depletion of both B- and T-lymphocytes, resulting in profound immunosuppression. Extending the time between induction therapy and alemtuzumab consolidation to allow for recovery of T-cells is a potential approach for future trials, if this strategy continues to be pursued. However, delaying consolidation places patients who have achieved PR at risk of relapse before consolidation treatment is initiated. Additionally, patients may be reluctant to consider additional consolidation therapy subsequent to a long observation period after induction therapy.
To determine whether future studies of alemtuzumab consolidation after chemoimmunotherapy are warranted, the effect of alemtuzumab on PFS after chemoimmunotherapy must be considered. Although alemtuzumab consolidation improved response rates and MRD negativity in our study, patients who received alemtuzumab did not experience better 2-year PFS or OS than patients who did not. Furthermore, the 2-year PFS of 72% in this study was similar to the 67% 2-year PFS in the previous CALGB 9712 study in which FR was administered.4 Longer follow-up is required to determine whether the improved CR and MRD-negative rates after alemtuzumab consolidation in this study will eventually translate into improved long-term survival. Given the toxicity and absence of clear survival benefit, CALGB will not pursue additional studies of alemtuzumab consolidation. Novel therapeutic agents such as lenalidomide, the cyclin-dependent kinase inhibitor flavopiridol (Alvocidib; sanofi-aventis, Paris, France), the phosphatidylinositol-3-kinase inhibitor CAL-101 (Calistoga Pharmaceuticals, Seattle, WA), and the syk inhibitor fostamatinib (Rigel Pharmaceuticals, South San Francisco, CA) are active in CLL and warrant clinical study as potential CLL consolidation therapies.24–29 Whether consolidation therapy improves long-term outcome in patients with CLL with detectable MRD after therapy is a research question; therefore, such therapy should not be administered outside of clinical trials.
Acknowledgment
We thank the patients who participated in this trial; the Cancer and Leukemia Group B member institutions, physicians, nurses, and clinical research associates who participated in this study; our protocol editor, Michael Kelly, MD; Helen Chen, MD, of the Cancer Therapy Evaluation Program at the National Cancer Institute; and Elizabeth Trehu, MD, of Genzyme.
Appendix
The following institutions participated in this study: University of Oklahoma, Oklahoma City, OK—Howard Ozer, MD, supported by Grant No. CA37447; Christiana Care Health Services, Wilmington, DE—Stephen Grubbs, MD, supported by Grant No. CA45418; Dana-Farber Cancer Institute, Boston, MA—Harold J. Burstein, MD, supported by Grant No. CA32291; Norris Cotton Cancer Center, Dartmouth Medical School, Lebanon, NH—Konstantin Dragnev, MD, supported by Grant No. CA04326; Georgetown University Medical Center, Washington, DC—Minetta C. Liu, MD, supported by Grant No. CA77597; Illinois Oncology Research Association, Peoria, IL—John W. Kugler, MD, supported by Grant No. CA35113; Kansas City Community Clinical Oncology Program, Kansas City, MO—Rakesh Gaur, MD; Mount Sinai Medical Center, Miami, FL—Rogerio C. Lilenbaum, MD, supported by Grant No. CA45564; North Shore–Long Island Jewish Health System, New Hyde Park, NY—Daniel Budman, MD, supported by Grant No. CA35279; Rhode Island Hospital, Providence, RI—William Sikov, MD, supported by Grant No. CA08025; Southeast Cancer Control Consortium, Goldsboro, NC—James N. Atkins, MD, supported by Grant No. CA45808; The Ohio State University Medical Center, Columbus, OH—Clara D. Bloomfield, MD, supported by Grant No. CA77658; University of California at San Diego, San Diego, CA—Barbara A. Parker, MD, supported by Grant No. CA11789; University of Chicago, Chicago, IL—Hedy L. Kindler, MD, supported by Grant No. CA41287; University of Iowa, Iowa City, IA—Daniel A. Vaena, MD, supported by Grant No. CA47642; University of Nebraska Medical Center, Omaha, NE—Anne Kessinger, MD, supported by Grant No. CA77298; University of North Carolina at Chapel Hill, Chapel Hill, NC—Thomas C. Shea, MD, supported by Grant No. CA47559; University of Vermont, Burlington, VT—Steven M. Grunberg, MD, supported by Grant No. CA77406; Wake Forest University School of Medicine, Winston-Salem, NC—David D. Hurd, MD, supported by Grant No. CA03927; Walter Reed Army Medical Center, Washington, DC—Brendan M. Weiss, MD, supported by Grant No. CA26806; Washington University School of Medicine, St Louis, MO—Nancy Bartlett, MD, supported by Grant No. CA77440; Western Pennsylvania Cancer Institute, Pittsburgh, PA—John Lister, MD.
Table A1.
Most Common Nonhematologic Toxicities (n = 102)
| Toxicity | Grade |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
2 |
3 |
4 |
5 |
||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | |
| FR induction | ||||||||||
| Fatigue | 48 | 47 | 29 | 28 | 5 | 5 | — | — | ||
| Nausea | 44 | 43 | 11 | 11 | — | — | — | |||
| Pain | 32 | 31 | 16 | 16 | 4 | 4 | — | — | ||
| Rash | 16 | 16 | 12 | 12 | 3 | 3 | — | — | ||
| Hyperglycemia | 17 | 17 | 8 | 8 | 1 | 1 | — | — | ||
| Transaminitis (SGOT) | 20 | 20 | 6 | 6 | — | — | — | |||
| Fever | 19 | 19 | 5 | 5 | — | — | — | |||
| Vomiting | 16 | 16 | 8 | 8 | — | — | — | |||
| Constipation | 18 | 18 | 4 | 4 | 1 | 1 | — | — | ||
| Diarrhea | 13 | 13 | 7 | 7 | 1 | 1 | — | — | ||
| Cough | 13 | 13 | 4 | 4 | — | — | — | |||
| Hypocalcemia | 13 | 13 | 3 | 3 | — | — | — | |||
| Serum creatinine | 13 | 13 | — | — | — | — | ||||
| Alemtuzumab consolidation (n = 58) | ||||||||||
| Fatigue | 19 | 33 | 12 | 21 | 10 | 17 | 1 | 2 | — | |
| Rash | 15 | 26 | 15 | 26 | — | — | — | |||
| Pain | 18 | 31 | 5 | 9 | 2 | 3 | — | — | ||
| Fever | 12 | 21 | 5 | 9 | — | — | — | |||
| Hyperglycemia | 12 | 21 | 5 | 9 | — | — | — | |||
| Nausea | 12 | 21 | 4 | 7 | — | — | — | |||
| Pruritus | 9 | 16 | 6 | 10 | 1 | 2 | — | — | ||
| Chills/rigors | 14 | 24 | 1 | 2 | — | — | — | |||
| Hypocalcemia | 9 | 16 | 4 | 7 | 1 | 2 | — | — | ||
| Cough | 9 | 16 | 2 | 3 | 3 | 5 | — | — | ||
| Transaminitis (SGOT) | 9 | 16 | — | 3 | 5 | — | — | |||
Abbreviations: FR, fludarabine plus rituximab; SGOT, serum glutamic oxaloacetic transaminase.
Footnotes
Supported in part by Grants No. CA31946, CA33601, and R21CA101332 (J.C.B.) from the National Cancer Institute, Bethesda, MD.
Presented in oral abstract form at the 51st Annual Meeting of the American Society of Hematology, December 5-8, 2009, New Orleans, LA, and 49th Annual Meeting of the American Society of Hematology, December 8-11, 2007, Atlanta, GA.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00098670
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Thomas S. Lin, GlaxoSmithKline (C) Consultant or Advisory Role: Thomas S. Lin, Bayer Pharmaceuticals (C), Genentech (C); Kanti R. Rai, Bayer Pharmaceuticals (C), Genentech (C); Brian K. Link, Genentech (C) Stock Ownership: None Honoraria: None Research Funding: Brian K. Link, Genentech Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Kathleen A. Donohue, John C. Byrd, Eva E. Hoke, Kanti R. Rai, Richard A. Larson
Administrative support: Thomas S. Lin, Kathleen A. Donohue, John C. Byrd, Margaret S. Lucas, Eva E. Hoke, Richard A. Larson
Provision of study materials or patients: Thomas S. Lin, John C. Byrd, Elizabeth M. Bengtson, Kanti R. Rai, James N. Atkins, Brian K. Link, Richard A. Larson
Collection and assembly of data: Thomas S. Lin, Kathleen A. Donohue, John C. Byrd, Margaret S. Lucas, Eva E. Hoke, Elizabeth M. Bengtson, Kanti R. Rai, James N. Atkins, Brian K. Link, Richard A. Larson
Data analysis and interpretation: Thomas S. Lin, Kathleen A. Donohue, John C. Byrd, Margaret S. Lucas, Eva E. Hoke, Kanti R. Rai,Richard A. Larson
Manuscript writing: Thomas S. Lin, Kathleen A. Donohue, John C. Byrd, Richard A. Larson
Final approval of manuscript: Thomas S. Lin, Kathleen A. Donohue, John C. Byrd, Margaret S. Lucas, Eva E. Hoke, Elizabeth M. Bengtson, Kanti R. Rai, James N. Atkins, Brian K. Link, Richard A. Larson
REFERENCES
- 1.Byrd JC, Murphy T, Howard RS, et al. Rituximab using a thrice weekly dosing schedule in B-cell chronic lymphocytic leukemia and small lymphocytic lymphoma demonstrates clinical activity and acceptable toxicity. J Clin Oncol. 2001;19:2153–2164. doi: 10.1200/JCO.2001.19.8.2153. [DOI] [PubMed] [Google Scholar]
- 2.O'Brien SM, Kantarjian H, Thomas DA, et al. Rituximab dose-escalation trial in chronic lymphocytic leukemia. J Clin Oncol. 2001;19:2165–2170. doi: 10.1200/JCO.2001.19.8.2165. [DOI] [PubMed] [Google Scholar]
- 3.Byrd JC, Peterson BL, Morrison VA, et al. Randomized phase 2 study of fludarabine with concurrent versus sequential treatment with rituximab in symptomatic, untreated patients with B-cell chronic lymphocytic leukemia: Results from Cancer and Leukemia Group B 9712 (CALGB 9712) Blood. 2003;101:6–14. doi: 10.1182/blood-2002-04-1258. [DOI] [PubMed] [Google Scholar]
- 4.Byrd JC, Rai K, Peterson BL, et al. Addition of rituximab to fludarabine may prolong progression-free survival and overall survival in patients with previoulsy untreated chronic lymphocytic leukemia: An updated retrospective comparative analysis of CALGB 9712 and CALGB 9011. Blood. 2005;105:49–53. doi: 10.1182/blood-2004-03-0796. [DOI] [PubMed] [Google Scholar]
- 5.Keating MJ, O'Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4079–4088. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 6.Tam CS, O'Brien S, Wierda W, et al. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008;112:975–980. doi: 10.1182/blood-2008-02-140582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hallek M, Fingerle-Rowson G, Fink AM, et al. Immunochemotherapy with fludarabine (F), cyclophosphamide (C), and rituximab (R) (FCR) versus fludarabine and cyclophosphamide (FC) improves response rates and progression-free survival (PFS) of previously untreated patients (pts) with advanced chronic lymphocytic leukemia (CLL) Blood. 2008;112(abstr 325) [Google Scholar]
- 8.O'Brien S, Moore JO, Boyd TE, et al. Randomized phase III trial of fludarabine plus cyclophosphamide with or without oblimersen sodium (Bcl-2 antisense) in patients with relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2007;25:1114–1120. doi: 10.1200/JCO.2006.07.1191. [DOI] [PubMed] [Google Scholar]
- 9.O'Brien SM, Kantarjian HM, Cortes J, et al. Results of the fludarabine and cyclophosphamide combination regimen in chronic lymphocytic leukemia. J Clin Oncol. 2001;19:1414–1420. doi: 10.1200/JCO.2001.19.5.1414. [DOI] [PubMed] [Google Scholar]
- 10.Keating MJ, Flinn I, Jain V, et al. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: Results of a large international study. Blood. 2002;99:3554–3561. doi: 10.1182/blood.v99.10.3554. [DOI] [PubMed] [Google Scholar]
- 11.Hillmen P, Skotnicki AB, Robak T, et al. Alemtuzumab compared with chlorambucil as first-line therapy for chronic lymphocytic leukemia. J Clin Oncol. 2007;25:5616–5623. doi: 10.1200/JCO.2007.12.9098. [DOI] [PubMed] [Google Scholar]
- 12.O'Brien SM, Kantarjian HM, Thomas DA, et al. Alemtuzumab as treatment for residual disease after chemotherapy in patients with chronic lymphocytic leukemia. Cancer. 2003;98:2657–2663. doi: 10.1002/cncr.11871. [DOI] [PubMed] [Google Scholar]
- 13.Moreton P, Kennedy B, Lucas G, et al. Eradication of minimal residual disease in B-cell chronic lymphocytic leukemia after alemtuzumab therapy is associated with prolonged survival. J Clin Oncol. 2005;23:2971–2979. doi: 10.1200/JCO.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 14.Wendtner CM, Ritgen M, Schweighofer CD, et al. Consolidation with alemtuzumab in patients with chronic lymphocytic leukemia (CLL) in first remission: Experience on safety and efficacy within a randomized multicenter phase III trial of the German CLL Study Group (GCLLSG) Leukemia. 2004;18:1093–1101. doi: 10.1038/sj.leu.2403354. [DOI] [PubMed] [Google Scholar]
- 15.Schweighofer CD, Ritgen M, Eichhorst BF, et al. Consolidation with alemtuzumab improves progression-free survival in patients with chronic lymphocytic leukaemia (CLL) in first remission: Long-term follow-up of a randomized phase III trial of the German CLL Study Group (GCLLSG) Br J Haematol. 2009;144:95–98. doi: 10.1111/j.1365-2141.2008.07394.x. [DOI] [PubMed] [Google Scholar]
- 16.Hainsworth JD, Vazquez ER, Spigel DR, et al. Combination therapy with fludarabine and rituximab followed by alemtuzumab in the first-line treatment of patients with chronic lymphocytic leukemia or small lymphocytic lymphoma: A phase 2 trial of the Minnie Pearl Cancer Research Network. Cancer. 2008;112:1288–1295 d. doi: 10.1002/cncr.23271. [DOI] [PubMed] [Google Scholar]
- 17.Byrd JC, Peterson BL, Rai KR, et al. Fludarabine followed by alemtuzumab consolidation for previously untreated chronic lymphocytic leukemia: Final report of Cancer and Leukemia Group B study 19901. Leuk Lymphoma. 2009;50:1589–1596. doi: 10.1080/10428190903150839. [DOI] [PubMed] [Google Scholar]
- 18.Montillo M, Tedeschi A, Miqueleiz S, et al. Alemtuzumab as consolidation after a response to fludarabine is effective in purging residual disease in patients with chronic lymphocytic leukemia. J Clin Oncol. 2006;24:2337–2342. doi: 10.1200/JCO.2005.04.6037. [DOI] [PubMed] [Google Scholar]
- 19.Cheson BD, Bennett JM, Grever M, et al. National Cancer Institute–sponsored Working Group guidelines for chronic lymphocytic leukemia: Revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–4997. [PubMed] [Google Scholar]
- 20.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 21.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 22.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–346. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan LD, Lee JY, Ambinder RF, et al. Rituximab does not improve clinical outcome in a randomized phase 3 trial of CHOP with or without rituximab in patients with HIV-associated non-Hodgkin lymphoma: AIDS-Malignancies Consortium Trial 010. Blood. 2005;106:1538–1543. doi: 10.1182/blood-2005-04-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chanan-Khan A, Miller KC, Musial L, et al. Clinical efficacy of lenalidomide in patients with relapsed or refractory chronic lymphocytic leukemia: Results of a phase II study. J Clin Oncol. 2006;24:5343–5349. doi: 10.1200/JCO.2005.05.0401. [DOI] [PubMed] [Google Scholar]
- 25.Ferrajoli A, Lee BN, Schlette EJ, et al. Lenalidomide induces complete and partial remissions in patients with relapsed and refractory chronic lymphocytic leukemia. Blood. 2008;111:5291–5297. doi: 10.1182/blood-2007-12-130120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin TS, Ruppert AS, Johnson AJ, et al. Phase II study of flavopiridol in relapsed chronic lymphocytic leukemia demonstrating high response rates in genetically high-risk disease. J Clin Oncol. 2009;27:6012–6018. doi: 10.1200/JCO.2009.22.6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedberg JW, Sharman J, Schaefer-Cutillo J, et al. Fostamatinib disodium (FosD), an oral inhibitor of Syk, is well-tolerated and has significant clinical activity in diffuse large B cell lymphoma (DLBCL) and chronic lymphocytic leukemia (SLL/CLL) Blood. 2008;112(abstr 3) [Google Scholar]
- 28.Flinn IW, Byrd JC, Furman RR, et al. Evidence of clinical activity in a phase I study of CAL-101, an oral p110 delta isoform-specific inhibitor of phosphatidylinositol 3-kinase, in patients with relapsed or refractory B-cell malignancies. Blood. 2009;114(abstr 380) [Google Scholar]
- 29.Byrd JC, Lin TS, Dalton JT, et al. Flavopiridol administered using a pharmacologically derived schedule is associated with marked clinical efficacy in refractory, genetically high-risk chronic lymphocytic leukemia. Blood. 2007;109:399–404. doi: 10.1182/blood-2006-05-020735. [DOI] [PMC free article] [PubMed] [Google Scholar]