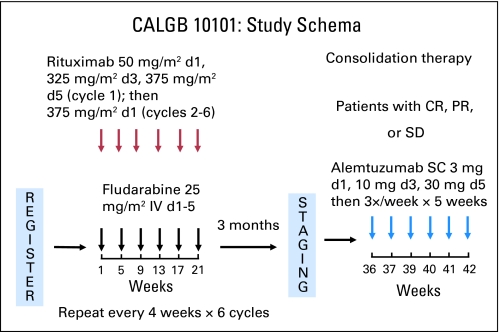
Fig 1.
Study schema for the CALGB (Cancer and Leukemia Group B) 10101 study. Patients received fludarabine 25 mg/m2 intravenously (IV) on days (d) 1 to 5 and rituximab 50 mg/m2 IV on d 1, 325 mg/m2 on d 3, and 375 mg/m2 on d 5 of cycle 1 and then 375 mg/m2 on d 1 of cycles 2 to 6; this was repeated every 28 d for 6 cycles. Three months after completing the final induction cycle, patients achieving stable disease (SD), partial response (PR), or complete response (CR) received alemtuzumab 3 mg subcutaneously (SC) on d 1, 10 mg on d 3, and 30 mg on d 5 and then 30 mg three times per week for 5 weeks (total dose, 493 mg).