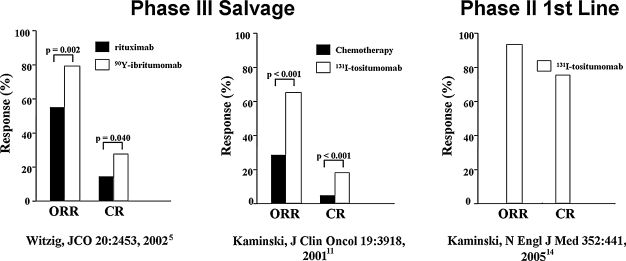
Figure 2.
Salvage and first-line radioimmunotherapy (RIT) in non-Hodgkin's lymphoma (NHL).5,11,14 Overall (ORR) and complete (CR) response rates in the pivotal phase III trial of 90Y-ibritumomab versus rituximab in relapsed/refractory low-grade, follicular, or transformed NHL (left). Patients randomized into the 90Y-ibritumomab arm were given a single therapeutic dose of 14.8 MBq/kg (0.4 mCi/kg) of 90Y-ibritumomab on day 7, preceded by 250 mg/m2 of rituximab. Patients randomized into the rituximab arm received rituximab 375 mg/m2 weekly × 4. The efficacy analysis showed an overall response rate (ORR) of 80% for 90Y-ibritumomab versus 56% for rituximab (p = 0.002). The complete remission (CR) was 30% for 90Y-ibritumomab versus 16% for rituximab (p = 0.04). Pivotal phase III trial of iodine-131 (131I)-tositumomab versus chemotherapy in low-grade, or transformed, NHL (middle). Patients who had not responded or had progressed after their most recent chemotherapy were treated with 131I-tositumomab at a dose contributing 75 cGy to the body, preceded by 450 mg of tositumomab. The patients had received a median of four prior chemotherapy regimens. Sixty-five percent (65%) of the patients had a response after 131I-tositumomab, compared with 28% after their last chemotherapy (p < 0.001); 20% of the patients had a complete remission after 131I-tositumomab, compared with 3% after their last chemotherapy (p < 0.001). Phase II first-line trial of 131I-tositumomab in stage III–IV follicular NHL. A single therapy dose of 131I-tositumomab (75 cGy to the body, preceded by 450 mg of tositumomab) led to ORRs and CRs of 95% and 75%, respectively, with very modest toxicity. Median progression-free survival was 6.1 years (graphics generated from data in Witzig et al., 5 Kaminski et al., 11 and Kaminski et al., 14 respectively).