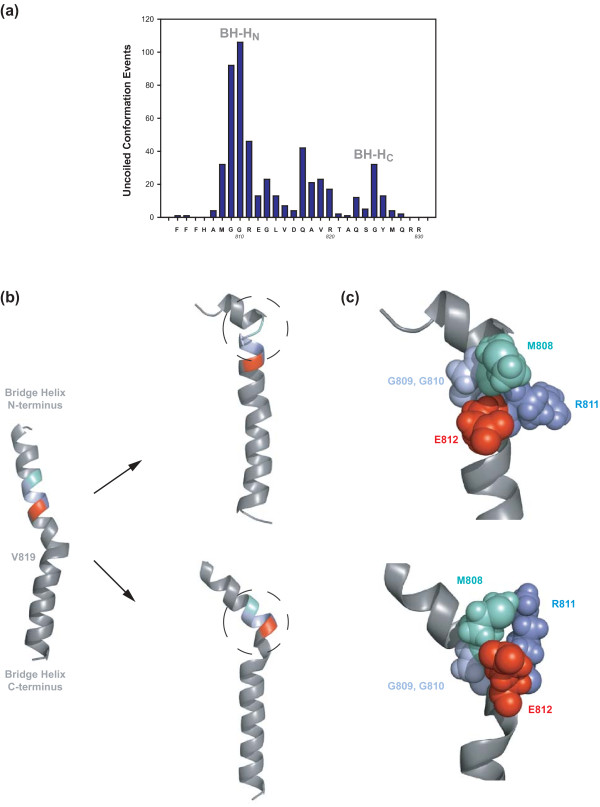
Figure 3.
Structural basis of BH-HN kinking. (A) Overview of local unfolding events incurred by a M. jannaschii Bridge Helix model during 27 independent 200 picosecond MD simulations. The number of 5 picosecond windows during which a specific part of the Bridge Helix adopts a 'coil' conformation are plotted against the Bridge Helix sequence shown on the horizontal axis. A major area of α-helical instability, BH-HN (centered on mjA'G810), is evident from this semi-quantitative analysis. Additional unstable regions include BH-HC (centered on mjA' G825) and a 'labile region' (spanning mjA'Q817 to R820; see Discussion for more details). (B) Examples of kinked BH-HN conformations arising from MD simulations. The Bridge Helix on the left represents the starting conformation as modelled on the yeast RNAPII structure PDB #2E2H. The minor bulge near the center of the Bridge Helix corresponds to the V819 position. During unrestrained simulation, different types of kinked BH-HN structures (circled) involving mjA' G809/G810 (pale blue) and M808 (pale green), R811 (blue) and E812 (red) side-chain interaction emerge stochastically. (C) Structural details of BH-HN kinking models. The relevant residues are shown in space-filling mode to illustrate spatial relationships. Two glycine residues, mjA' G809 and G810 (pale blue) form a highly flexible hinge that allows M808 (pale green) to interact extensively via van der Waals interactions with the side-chains of R811 (blue) and E812 (red). In some cases these interactions create a stretched 310 helix immediately C-terminal to R811 and E812.