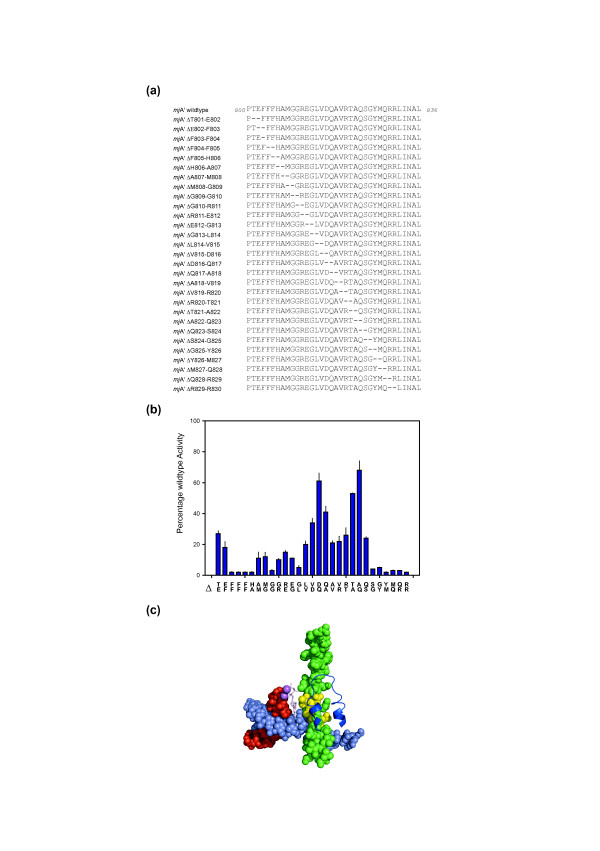
Figure 4.
Creation of locally twisted Bridge Helix structures. (A) Sequences of the deletion constructs. A two-amino acid deletion window is moved systematically in a single residue step through the entire Bridge Helix primary sequence. The deletions remove two adjacent residues, but, more importantly, join the sequences bordering the mutation with 180° twist because of the removal of half an α-helical turn. (B) Activity of Bridge Helix mutants containing two-amino acid deletions shown relative to wildtype activity (100%). The amino acid pairs deleted from the primary sequence are shown vertically along the horizontal axis. Two distinct peaks of relative insensitivity to the deletions centered on mjA' ΔD816/Q817 and mjA' ΔA822/Q823 are discernible. All assays were performed in at least quadruplicate, with error bars showing standard deviation from the average value. (C) Position of the deletion-insensitive region of the Bridge Helix relative to other elements of the catalytic site. The Bridge Helix and other structures are shown using the same color-scheme as used in Figure 1A (yeast RNAPII elongation complex; [PDB #2E2H]). Residues orthologous to residues displaying the highest activity (>50%) levels in the two-amino acid scan (mjA' D816, Q817, T821, A822 and Q823) are highlighted in yellow.