Abstract
Reverse total shoulder arthroplasty (RTSA) has been reported to be associated with a complication rate that is four times that of conventional total shoulder arthroplasty. It is the purpose of this article to identify and understand the most common and most serious complications of RTSA and to review current methods of prevention and treatment. The current literature was reviewed to identify type and prevalence of reported complications and to identify risk factors, preventive measures as well as technical details for management strategies for complications of RTSA. The variable accuracy of reporting and the heterogeneity of methodology in the literature limited our study, however, a definitive ranking of most to least common complication emerged. The currently identified most common complication is scapular notching. The clinically most relevant complications are infection, instability and acromial fractures. Haematoma formation used to be very frequent but can be controlled, glenoid component loosening, however, is rare when compared with conventional total shoulder replacement. In conclusion, RTSA is associated with a high rate of complications. Their incidence and the results of their treatment are inconsistently reported. To document and then prevent complications, a standardised monitoring tool including clear definitions and assessment instructions appears necessary.
Background
Reversal of the physiological ball and socket configuration of the humerus and glenoid results in medialisation and distalisation of the centre of rotation of the shoulder joint and increases the deltoid muscle moment arm thereby allowing recruitment of more deltoid fibres for elevation and abduction. This feature is unique to the reverse total shoulder arthroplasties (RTSA) and valuable [1, 2] particularly for treatment of rotator cuff tear arthropathy [3–8], massive irreparable rotator cuff tears without osteoarthritis and failed hemiarthroplasty with irreparable rotator cuff tearing [9, 10]. This clinically successful concept, however, implies changes of joint physiology and biomechanics [4, 11] and might increase the potential for complications. Precise knowledge of the probability and the implications of the various complications for a given indication would be imperative for judicious use of RTSA. Frequent complications have been well described [12]; the studies in the literature, however, are heterogeneous (e.g. different indications, different prostheses, and different populations) and definitions and requirements for reporting of complications are non-existent. A meta-analysis of currently available data would therefore be unreliable. For the purpose of this study we present complications in the order of frequency of mention in the literature and in two personal series: a first series of 111 cases involving Delta III® (DEPUY) and a second series of 230 cases involving Anatomical Reverse® (ZIMMER, Inc.) prostheses. Complications were identified in the order of frequency (Table 1). The most frequent complication is scapular notching followed by complications with the glenoid component (e.g. loosening). Haematoma, infections and instability are reported more frequently than neurological complications, fractures of the acromion and complications of the humeral component.
Table 1.
Complications of reverse total shoulder arthroplasty (RTSA) found in the literature
| Study | Patient number | Mean follow-up (months) | Haematoma | Infections | Instability | Scapular notching | Glenoidal complications | Humeral complications | Fracture of the acromion | Neurological complications | Prosthesis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gilbart and Gerber, unpublished data | 111 | 26 | 17 (15%) | 1 (1%) | 7 (6%) | 21 (19%) | 5 (5%) | 6 (5%) | 4 (4%) | Delta III | |
| Molé and Favard [1], 2006 | 527 | 14 (3%) | 27 (5%) | 18 (3.4%) | 27 (5%) | 11 (2%) | 16 (3%) | 6 (1%) | Mostly Delta | ||
| Gerber et al. unpublished data (series 2005–2009) | 230 | 22.3 | 5 (2.1%) | 2 (0.8%) | 4 (1.7%) | 115 (50%) | 7 (3%) | 1 (0.4%) | 5 (2.2%) | 11 (4.7%) | Anatomical Inverse |
| Levy et al. [48], 2007 | 29 | 29 | 1 (3%) | 4 (14%) | 1 (3%) | 1 (3%) | 11 (38%a) | Reverse Shoulder System | |||
| Levy et al. [10], 2007 | 19 | 44 | 1 (5%) | 1 (5%) | 19 (100%) | 3 (15%) | 2 (10%) | 3 (15%) | Encore | ||
| Guery et al. [50], 2006 | 71 | 70 | 3 (4%) | 2 (3%) | 3 (4%) | Delta III | |||||
| Werner et al. [13], 2005 | 58 | 38 | 12 (21%) | 2 (3%) | 5 (9%) | 56 (96%) | 3 (5%) | 1 (2%) | 4 (7%) | Delta III | |
| Boileau et al. [2], 2005 | 45 | 40 | 2 (4%) | 24 (53%) | 1 (2%) | 3 (7%) | Delta III | ||||
| Frankle et al. [51], 2005 | 60 | 71 | 2 (3%) | 0 (0%) | 1 (2%) | 1 (2%) | 2 (3%) | Reverse Shoulder System | |||
| Klein et al. [52], 2008 | 20 | 33 | 2 (10%) | 1 (5%) | 1 (5%) | 0 (0%) | 0 (0%) | 0 (0%) | Delta III | ||
| Grassi et al. [15], 2009 | 23 | 26 | (0%) | 1 (4%) | 16 (70%) | 2 (9%) | 0 (0%) | Delta III | |||
| Sirveaux et al. [14], 2004 | 73 | 80 | 1 (1%) | 49 (67%) | 12 (16%) | Delta III |
Bold numbers are the two most common reported complications in one study. Other less frequent complications are not included in the table.
aIndication for RTSA was humeral fractures in this study; Neurological complications consisted mostly of radial nerve palsy
Complications
Scapular notching
The most frequently reported “complication” of RTSA is notching of the bone of the inferior and posterior scapular neck [4, 13–17] (Fig. 1). Notching identifies the radiographic appearance of resorption or wear of the lateral pillar of the scapula immediately medial and progressively also superior to the inferior aspect of the glenoid baseplate. Its severity has been stratified by the Nérot classification [18] starting with no visible notching on true anteroposterior radiographs (stage 0) to a resorption which goes beyond the central peg of the glenoid component (stage 4). Although scapular notching is clearly an anatomical complication with partial destruction of the inferior aspect of the glenoid, its clinical relevance is uncertain; in some studies it was clearly associated with an inferior clinical outcome [19] and in others it was considered to be clinically irrelevant [20]. In a remarkable case study, Nyffeler et al. could document that the superior baseplate remained very solidly bound to the underlying bone even though the inferior half of the glenoid had been resorbed [21]. This observation tallies with the fact that revision for notching or for loosening secondary to notching is essentially unreported.
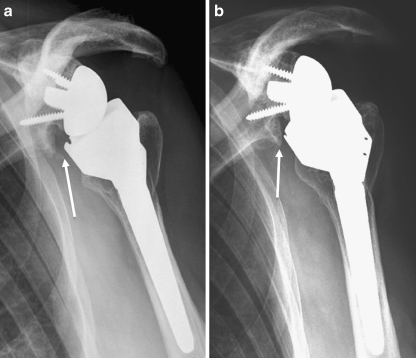
Fig. 1.
Radiological anteroposterior view of scapular notching grade 2 (according to Nérot classification) at three (a) and eight years (b) after reverse total shoulder arthroplasty (RTSA) (Delta III)
Several preoperative findings have been associated with development of notching; rotator cuff tear arthropathy, fatty infiltration of the infraspinatus, narrowed acromiohumeral distance and a superiorly oriented glenoid are risk factors for developing notching [9]. Operative parameters associated with notching are the anterosuperior approach, high position of the baseplate [9, 22] and inadequate prosthesis-scapular neck angle [19]. Inferior positioning of the glenoid baseplate is probably not only imperative to obtain good range of movement but also the most important factor to prevent scapular notching [22].
Prediction of the likelihood of notching with a sensitivity of 91% and specificity of 88% can be achieved by using the notching index, calculated from the height of implantation of the glenosphere and the postoperative prosthesis-scapular neck angle [19].
Design parameters of the prosthesis used may be underestimated. A prosthesis which lateralises the centre of rotation is likely to lead to less notching, and a prosthesis which medialises the centre of rotation is likely to lead to more notching [23]. Further, a prosthesis with a high stability index, e.g. a deep concave component, will lead to more notching than a shallow concave component [24].
Currently, the safest methods to prevent notching are inferior positioning of the glenoid baseplate, larger size implants with shallow concave components [7, 24] and, possibly, use of a prosthetic system with less medialisation of the centre of rotation [23].
Complications of the glenoid component
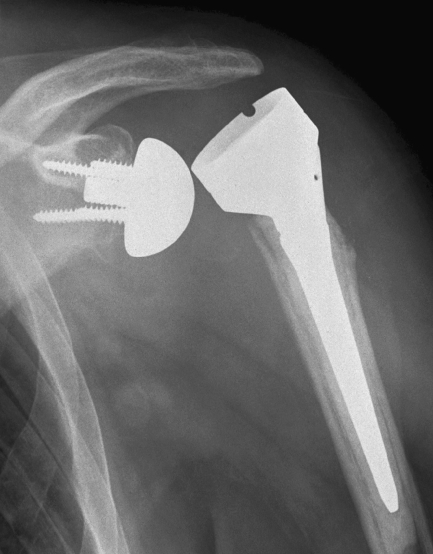
Reported glenoid complications include glenoid loosening (Fig. 2), glenoid component dissociation, unscrewing and scapular neck fracture. Glenoid loosening is the most frequent glenoid component complication in RTSA but it is distinctly less frequent than glenoid component loosening in conventional TSA [25, 26]. Loosening has been reported as the most common need for revision [27]. Its prevalence has been documented to be 4.1% after two years, excluding the prostheses which had been removed before a two-year follow-up [1].
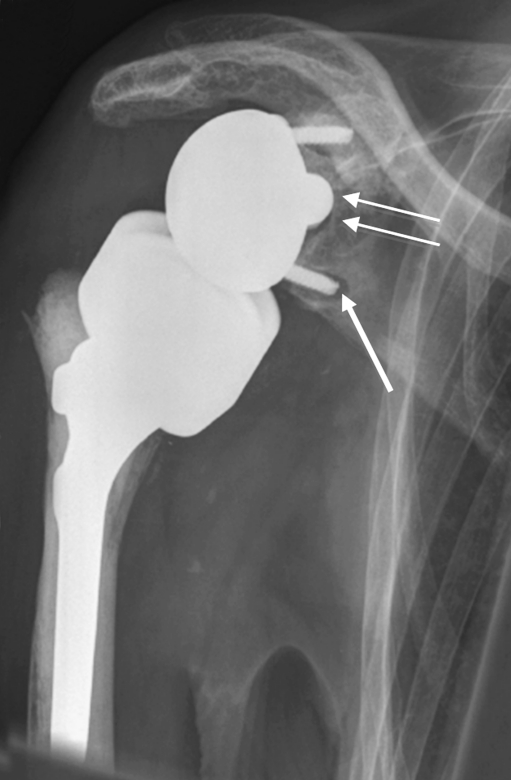
Fig. 2.
Glenoid component loosening six months after reverse total shoulder arthroplasty (RTSA) for irreparable rotator cuff arthropathy and osteoarthritis. Radiolucency is seen particularly around the inferior screw (arrow) and around the glenoidal implant central peg (double arrow)
Risk factors for glenoid loosening are female gender, age younger than 70 years and a superolateral approach [1]. Superior tilt, often associated with the superolateral approach, appears as a risk factor for glenoid loosening in both of our series.
Besides specific designs of different reversed prosthesis [28], biomechanical experiments suggest elements of surgical technique of glenoid fixation play key roles. Accurate placement of the inferior screw in good quality bone [29] (e.g. not excessively reamed glenoid) and caudal positioning [22] have been identified as protective or advantageous with regard to primary stability and range of motion, respectively. Scapular notching has been associated with glenoid loosening [30]; this, however, has not yet been confirmed as a reliable, clinical risk factor [21, 31].
Infections have also been linked to loosening [1], but are considered to be a different problem. They are mentioned because infection should always be ruled out before or during revision of a loose glenoid component.
Once established, treatment of loosening might become challenging. The guiding parameter for decision-making is the remaining scapular bone stock. Simple removal of the loose implant is effective in relieving pain but does not improve shoulder function. Direct glenoid component re-implantation requires sufficient glenoid bone stock [32]. This bone stock can be constructed with an iliac bone graft plus a base plate with a lengthened central peg [33] or with a staged repair, with autogenous cortico-cancellous bone grafting of the glenoid cavity [32], awaiting integration of the implanted bone using a hemiarthroplasty configuration and re-implantation of a glenoid component after six to nine months [7]. It is currently not established which of the two treatment regimens is superior in well defined clinical situations.
Haematoma
Incidence of haematoma alone, although relatively common, does not affect the overall outcome of RTSA [1]. This might be contributing to a potential underreporting in the currently available studies. No specific risk factor could be associated with development of haematoma [1]. Haematoma formation is, however, a substantial risk factor for the development of infection [34]. As infection is very frequent in RTSA, great care should be made to prevent haematoma formation. Whether fibrin sealants [7] or suction drainage are truly helpful is not established. If a major haematoma forms, surgical evacuation and lavage of the joint should be considered.
Infection
Infection is reported to be less frequent in conventional TSA than in knee or hip arthroplasty [35]. Conversely, the incidence of infections after primary RTSA is around 5% (Table 1), thus substantially higher than in conventional TSA and similar to hip or knee arthroplasty [31, 35]. The reasons for the extraordinarily high infection rate are multifactorial. The large dead space caused by the reverse configuration of the joint has been accused. The arthroplasty is not surrounded and covered by living tissue in the absence of musculotendinous rotator cuff tissue. Patient related factors are advanced age [1] and multiple previous operations [36]. Those series with many revision revised rather than primary replacements report higher infection rates [2]. Perioperative factors associated with increased rate of infection are haematoma [34] and lack of perioperative antibiotic prophylaxis.
In the series of Molé and Favard [1], the most commonly identified organism was propionibacterium acnes. This bacterium typically produces late, chronic, relatively low grade infections, colonisation of loose prostheses and, exceptionally, acute postoperative infections [37]. It is likely to be present in more revision cases than suspected [38].
Infection of a RTSA should be considered to be present in a patient with persistent pain, a CRP over 10 mg/l and/or ESR over 30 mm/h. In an analogy to total knee replacements, joint fluid aspirates with more than 1100/mm2 and over 64% of neutrophils are considered to be proof of infection until proven otherwise [39]. For shoulder prosthesis, this criteria however might not be sensitive enough [40].
It has been recommended that not only proven but also suspected infections should be revised operatively [41]. Perioperative antibiotic prophylaxis should not be withheld for revision surgery if infection is considered [42] since it appears not to affect the sensitivity of intraoperative cultures substantially. Treatment of acute infection using antibiotics and debridement may be effective [1]. Debridement with retention, irrigation and suction and intravenous antibiotics is a choice for infections with symptoms less than three weeks in a stable prosthesis and no growth on preoperative cultures [41]. This regimen, however, is ineffective in chronic or late infections [1, 36]. There is not enough evidence to definitively suggest two-staged over single stage revision surgery for infected shoulder prosthesis, particularly for RTSA, but there appears to be a tendency to use a staged approach for late infections (Fig. 3) with debridement, implantation of an antibiotic coated spacer and late re-implantation of RTSA [1, 43] (Fig. 4).
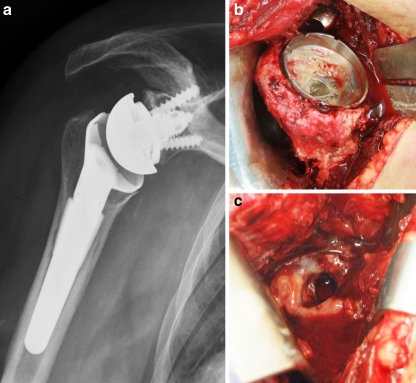
Fig. 3.
Radiological anteroposterior view (a) and intraoperative views (b, c) of an infected reverse total shoulder arthroplasty (RTSA)
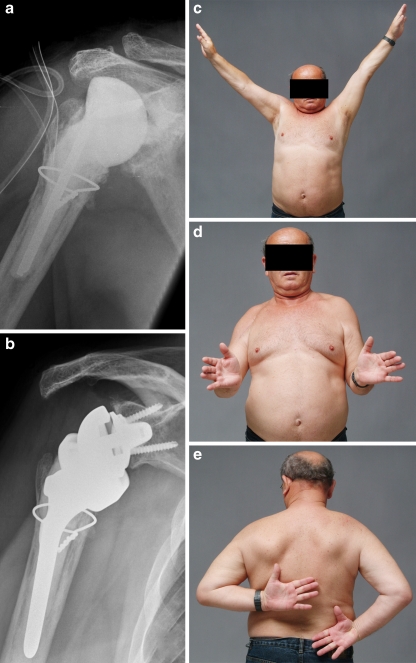
Fig. 4.
Staged treatment of infected reverse total shoulder arthroplasty (RTSA). Radiological anteroposterior view of implanted cement spacer (a) as treatment for an infected RTSA (Fig. 3) and late re-implantation of RTSA (b) with reasonable clinical results (c–e)
Instability
Instability at the interface of ball and socket is usually detected at its full range, which is dislocation (Fig. 5). The incidence of instability of RTSA ranges from 0 to 14% in heterogenic studies with mostly limited number of cases and different definition of instability. However, in large less heterogenic series, 3.4% of RTSA were unstable and this exclusively in the anterior and anterolateral direction [1]. Biomechanically, lack of compressive forces followed by shallow socket depth are the main parameters associated with instability [44]. Small glenoid size, deltopectoral as opposed to superolateral approach and poor subscapularis muscle (higher than Goutallier grade 3) have also been associated with increased risk of instability [1]. Further, ensuring adequate humeral length [45] is likely to decrease instability of RTSA. Most cases of dislocations occur during the first few months after implantation. In such cases surgical error appears likely and closed reduction alone is often associated with recurrence. Late dislocations (more than one year after implantation) can in most cases be successfully treated by closed reduction [1] without recurrence.
Fig. 5.
Anteroposterior view of a reverse total shoulder arthroplasty (RTSA) with anterolateral dislocation
Fracture of the acromion
If a RTSA is indicated, the acromion is usually eroded from the underlying head, brittle or potentially already fractured. If the subscapularis muscle is intact, the erosion is posterior and may involve the scapular spine. After reconstruction with a RTSA, the arm is lengthened by approximately 2.5 cm over normal length. This implies that the tension of the deltoid increases. In addition, with the substantially longer lever arm of the deltoid due to medialisation of the centre of rotation, loads on the acromion increase. Fracture of the acromion is therefore not surprising and was found to occur in 3% of the 527 RTSA investigated by Molé and Favard [1]. Potential risk factors were a deltopectoral approach [1] and high tension of the deltoid muscle produced by excessive lateralisation and humeral lengthening. The best treatment option for acromial fractures after RTSA remains uncertain [46]. It appears, however, that the fractures of the scapular spine have to be interpreted very differently [46]. Whereas acromial fractures can be treated conservatively without major dysfunction of the shoulder, this is not the case for spine fractures that result in displacement, pain and dysfunction and may require open reduction and internal fixation [46].
Other complications
Poor screw placement was identified as the most common intraoperative complication, followed by medial vault penetration, too large central glenoid hole, glenoid fracture, calcar fractures and humeral shaft fracture. Higher intraoperative complication rates have been reported recently [47]. Early postoperative complications include heterotopic ossification [12].
Potentially relevant neurological complications involving the brachial plexus or the axillary nerve are rare and mostly reversible during the first three postoperative months [1]. If monitored, other neurological complications such as radial nerve palsy might be more frequent in RTSA in management of humerus fractures [48].
Partial disengagement of the glenosphere seems not to be associated with poor functional outcome in the early postoperative stage [1, 49]; but if complete, requires revision surgery [49].
Humeral complications include fractures as the most common contributor, followed by migration and loosening. The latter is almost always associated with another complication such as infection or instability [1].
Discussion
The very high complication rate of RSTA is currently accepted because the enormous improvement of shoulder function and the quality of life following implantation for appropriate indications. Furthermore, if the prosthesis can be retained during the treatment of any complication, the outcome is still remarkable.
All complications, which require removal of the implant, however, leave the patient with very poor function. Therefore, prevention of all the complications likely to lead to removal of the implant should be studied in detail. Currently we lack definitions of complications and standards of reporting adverse events; prospective studies with clearly documented outcome parameters are at best, rare. A reasonable meta-analysis of the currently available data seems therefore worthless. We are left with careful analysis and interpretation of available evidence to illuminate and understand the most common complications, their potential risk factors and reasonable management strategies.
The lack of large series such as the one reported by Molé and Favard [1] impedes progress. Specifically, we have only very limited series relating to RTSA in specific indications but we need to know how likely each complication is in a specific patient and not in completely different circumstances. We feel that the frequent use of RTSA should mandate the orthopaedic community to introduce a common reporting tool to monitor the true results and complications of RTSA.
Contributor Information
Mazda Farshad, Phone: +41-44-3865752, Email: mazda.farshad@balgrist.ch.
Christian Gerber, Email: Christian.Gerber@balgrist.ch.
References
- 1.Molé D, Favard L. Excentered scapulohumeral osteoarthritis. Rev Chir Orthop Reparatrice Appar Mot. 2007;93(6 Suppl):37–94. doi: 10.1016/s0035-1040(07)92708-7. [DOI] [PubMed] [Google Scholar]
- 2.Wall B, Nove-Josserand L, O'Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476–1485. doi: 10.2106/JBJS.F.00666. [DOI] [PubMed] [Google Scholar]
- 3.Boileau P, Gonzalez JF, Chuinard C, Bicknell R, Walch G. Reverse total shoulder arthroplasty after failed rotator cuff surgery. J Shoulder Elbow Surg. 2009;18(4):600–606. doi: 10.1016/j.jse.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 Suppl S):147S–161S. doi: 10.1016/j.jse.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Boulahia A, Edwards TB, Walch G, Baratta RV. Early results of a reverse design prosthesis in the treatment of arthritis of the shoulder in elderly patients with a large rotator cuff tear. Orthopedics. 2002;25(2):129–133. doi: 10.3928/0147-7447-20020201-16. [DOI] [PubMed] [Google Scholar]
- 6.Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am. 2008;90(6):1244–1251. doi: 10.2106/JBJS.G.00775. [DOI] [PubMed] [Google Scholar]
- 7.Gerber C, Pennington SD, Nyffeler RW. Reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2009;17(5):284–295. doi: 10.5435/00124635-200905000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Vanhove B, Beugnies A. Grammont’s reverse shoulder prosthesis for rotator cuff arthropathy. A retrospective study of 32 cases. Acta Orthop Belg. 2004;70(3):219–225. [PubMed] [Google Scholar]
- 9.Levigne C, Boileau P, Favard L, Garaud P, Molé D, Sirveaux F, et al. Scapular notching in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2008;17(6):925–935. doi: 10.1016/j.jse.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Levy JC, Virani N, Pupello D, Frankle M. Use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty in patients with glenohumeral arthritis and rotator cuff deficiency. J Bone Joint Surg Br. 2007;89(2):189–195. doi: 10.2106/JBJS.E.01310. [DOI] [PubMed] [Google Scholar]
- 11.Kontaxis A, Johnson GR. The biomechanics of reverse anatomy shoulder replacement—a modelling study. Clin Biomech (Bristol) 2009;24(3):254–260. doi: 10.1016/j.clinbiomech.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Wierks C, Skolasky RL, Ji JH, McFarland EG. Reverse total shoulder replacement: intraoperative and early postoperative complications. Clin Orthop Relat Res. 2009;467(1):225–234. doi: 10.1007/s11999-008-0406-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87(7):1476–1486. doi: 10.2106/JBJS.D.02342. [DOI] [PubMed] [Google Scholar]
- 14.Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86(3):388–395. doi: 10.1302/0301-620X.86B3.14024. [DOI] [PubMed] [Google Scholar]
- 15.Grassi FA, Murena L, Valli F, Alberio R. Six-year experience with the Delta III reverse shoulder prosthesis. J Orthop Surg (Hong Kong) 2009;17(2):151–156. doi: 10.1177/230949900901700205. [DOI] [PubMed] [Google Scholar]
- 16.Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17–22. doi: 10.1067/mse.2001.110515. [DOI] [PubMed] [Google Scholar]
- 17.John M, Pap G, Angst F, Flury MP, Lieske S, Schwyzer HK et al. Short-term results after reversed shoulder arthroplasty (Delta III) in patients with rheumatoid arthritis and irreparable rotator cuff tear. Int Orthop 34(1):71–77 [DOI] [PMC free article] [PubMed]
- 18.Valenti P, Boutens D, Nerot C. Delta 3 reversed prosthesis for osteoarthritis with massive rotator cuff tear: long term results (>5 years) In: Walch G, Boileau P, Molé D, editors. Shoulder prosthesis. Montpellier: Sauramps Medical; 2001. pp. 253–259. [Google Scholar]
- 19.Simovitch RW, Zumstein MA, Lohri E, Helmy N, Gerber C. Predictors of scapular notching in patients managed with the Delta III reverse total shoulder replacement. J Bone Joint Surg Am. 2007;89(3):588–600. doi: 10.2106/JBJS.F.00226. [DOI] [PubMed] [Google Scholar]
- 20.Levigne C, Boileau P, Favard L (2006) Scapular notching. In: Walch G, Boileau P, Molé D (eds) Reverse shoulder arthroplasty: clinical results, complications, revision. Sauramps Medical, Montpellier, France, pp 353–372
- 21.Nyffeler RW, Werner CM, Simmen BR, Gerber C. Analysis of a retrieved delta III total shoulder prosthesis. J Bone Joint Surg Br. 2004;86(8):1187–1191. doi: 10.1302/0301-620X.86B8.15228. [DOI] [PubMed] [Google Scholar]
- 22.Nyffeler RW, Werner CM, Gerber C. Biomechanical relevance of glenoid component positioning in the reverse Delta III total shoulder prosthesis. J Shoulder Elbow Surg. 2005;14(5):524–528. doi: 10.1016/j.jse.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 23.Gutierrez S, Levy JC, Frankle MA, Cuff D, Keller TS, Pupello DR, et al. Evaluation of abduction range of motion and avoidance of inferior scapular impingement in a reverse shoulder model. J Shoulder Elbow Surg. 2008;17(4):608–615. doi: 10.1016/j.jse.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Gutierrez S, Luo ZP, Levy J, Frankle MA. Arc of motion and socket depth in reverse shoulder implants. Clin Biomech (Bristol) 2009;24(6):473–479. doi: 10.1016/j.clinbiomech.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Wirth MA, Rockwood CA., Jr Complications of total shoulder-replacement arthroplasty. J Bone Joint Surg Am. 1996;78(4):603–616. doi: 10.2106/00004623-199604000-00018. [DOI] [PubMed] [Google Scholar]
- 26.Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg. 2002;11(2):130–135. doi: 10.1067/mse.2002.121146. [DOI] [PubMed] [Google Scholar]
- 27.Fevang BT, Lie SA, Havelin LI, Skredderstuen A, Furnes O. Risk factors for revision after shoulder arthroplasty: 1,825 shoulder arthroplasties from the Norwegian Arthroplasty Register. Acta Orthop. 2009;80(1):83–91. doi: 10.1080/17453670902805098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harman M, Frankle M, Vasey M, Banks S. Initial glenoid component fixation in “reverse” total shoulder arthroplasty: a biomechanical evaluation. J Shoulder Elbow Surg. 2005;14(1 Suppl S):162S–167S. doi: 10.1016/j.jse.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 29.Chebli C, Huber P, Watling J, Bertelsen A, Bicknell RT, Matsen F., 3rd Factors affecting fixation of the glenoid component of a reverse total shoulder prothesis. J Shoulder Elbow Surg. 2008;17(2):323–327. doi: 10.1016/j.jse.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 30.Cazeneuve JF, Cristofari DJ. Grammont reversed prosthesis for acute complex fracture of the proximal humerus in an elderly population with 5 to 12 years follow-up. Rev Chir Orthop Reparatrice Appar Mot. 2006;92(6):543–548. doi: 10.1016/s0035-1040(06)75911-6. [DOI] [PubMed] [Google Scholar]
- 31.Seebauer L. Total reverse shoulder arthroplasty: European lessons and future trends. Am J Orthop (Belle Mead NJ) 2007;36(12 Suppl 1):22–28. [PubMed] [Google Scholar]
- 32.Neyton L, Sirveaux F, Roche O, Molé D, Boileau P, Walch G. Results of revision surgery for glenoid loosening: a multicentric series of 37 shoulder prosthesis. Rev Chir Orthop Reparatrice Appar Mot. 2004;90(2):111–121. doi: 10.1016/s0035-1040(04)70032-x. [DOI] [PubMed] [Google Scholar]
- 33.Hill JM, Norris TR. Long-term results of total shoulder arthroplasty following bone-grafting of the glenoid. J Bone Joint Surg Am. 2001;83-A(6):877–883. [PubMed] [Google Scholar]
- 34.Cheung EV, Sperling JW, Cofield RH. Infection associated with hematoma formation after shoulder arthroplasty. Clin Orthop Relat Res. 2008;466(6):1363–1367. doi: 10.1007/s11999-008-0226-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fehringer EV, Mikuls TR, Michaud KD, Henderson WG, O'Dell JR. Shoulder arthroplasties have fewer complications than hip or knee arthroplasties in US veterans. Clin Orthop Relat Res 468(3):717–722 [DOI] [PMC free article] [PubMed]
- 36.Coste JS, Reig S, Trojani C, Berg M, Walch G, Boileau P. The management of infection in arthroplasty of the shoulder. J Bone Joint Surg Br. 2004;86(1):65–69. [PubMed] [Google Scholar]
- 37.Zeller V, Ghorbani A, Strady C, Leonard P, Mamoudy P, Desplaces N. Propionibacterium acnes: an agent of prosthetic joint infection and colonization. J Infect. 2007;55(2):119–124. doi: 10.1016/j.jinf.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 38.Kelly JD, 2nd, Hobgood ER. Positive culture rate in revision shoulder arthroplasty. Clin Orthop Relat Res. 2009;467(9):2343–2348. doi: 10.1007/s11999-009-0875-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghanem E, Parvizi J, Burnett RS, Sharkey PF, Keshavarzi N, Aggarwal A, et al. Cell count and differential of aspirated fluid in the diagnosis of infection at the site of total knee arthroplasty. J Bone Joint Surg Am. 2008;90(8):1637–1643. doi: 10.2106/JBJS.G.00470. [DOI] [PubMed] [Google Scholar]
- 40.Topolski MS, Chin PY, Sperling JW, Cofield RH. Revision shoulder arthroplasty with positive intraoperative cultures: the value of preoperative studies and intraoperative histology. J Shoulder Elbow Surg. 2006;15(4):402–406. doi: 10.1016/j.jse.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 41.Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351(16):1645–1654. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]
- 42.Ghanem E, Parvizi J, Clohisy J, Burnett S, Sharkey PF, Barrack R. Perioperative antibiotics should not be withheld in proven cases of periprosthetic infection. Clin Orthop Relat Res. 2007;461:44–47. doi: 10.1097/BLO.0b013e318065b780. [DOI] [PubMed] [Google Scholar]
- 43.Sperling JW, Kozak TK, Hanssen AD, Cofield RH. Infection after shoulder arthroplasty. Clin Orthop Relat Res. 2001;382:206–216. doi: 10.1097/00003086-200101000-00028. [DOI] [PubMed] [Google Scholar]
- 44.Gutierrez S, Keller TS, Levy JC, Lee WE, 3rd, Luo ZP. Hierarchy of stability factors in reverse shoulder arthroplasty. Clin Orthop Relat Res. 2008;466(3):670–676. doi: 10.1007/s11999-007-0096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ladermann A, Williams MD, Melis B, Hoffmeyer P, Walch G. Objective evaluation of lengthening in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(4):588–595. doi: 10.1016/j.jse.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 46.Walch G, Mottier F, Wall B, Boileau P, Molé D, Favard L. Acromial insufficiency in reverse shoulder arthroplasties. J Shoulder Elbow Surg. 2009;18(3):495–502. doi: 10.1016/j.jse.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 47.Flury MP, Frey P, Goldhahn J, Schwyzer HK, Simmen BR (2010) Reverse shoulder arthroplasty as a salvage procedure for failed conventional shoulder replacement due to cuff failure-midterm results. Int Orthop. doi:10.1007/s00264-010-0990-z [DOI] [PMC free article] [PubMed]
- 48.Levy J, Frankle M, Mighell M, Pupello D. The use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty for proximal humeral fracture. J Bone Joint Surg Am. 2007;89(2):292–300. doi: 10.2106/JBJS.E.01310. [DOI] [PubMed] [Google Scholar]
- 49.Middernacht B, Wilde L, Molé D, Favard L, Debeer P. Glenosphere disengagement: a potentially serious default in reverse shoulder surgery. Clin Orthop Relat Res. 2008;466(4):892–898. doi: 10.1007/s11999-007-0090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G (2006) Reverse total shoulder arthroplasty. Survivorship analysis of eight replacements followed for ten years. J Bone Joint Surg Am 88(8):1742–1747 [DOI] [PubMed]
- 51.Frankle M, Siegal S, Pupello D, Saleem A, Mighell M, Vasey M (2005) The Reverse Shoulder Prosthesis for glenohumeral arthritis associated with severe rotator cuff dificiency. A minimum two-year follow-up study of sixty patients. J Bone Joint Surg Am 87(8):1697–1705 [DOI] [PubMed]
- 52.Klein M, Juschka M, Hinkenjann B, Scherger B, Ostermann PA (2008) Treatment of comminuted fractures of the proximal humerus in elderly patients with the Delta III reverse shoulder prosthesis. J Orthop Trauma, 22(10):698–704 [DOI] [PubMed]