Figure 5.
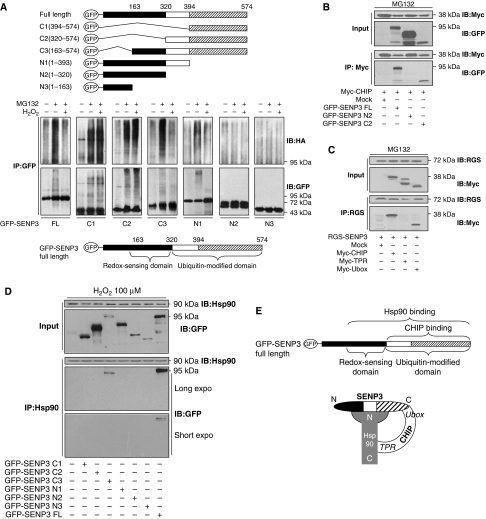
SENP3 interplays with CHIP and Hsp90 in a sophisticated way. (A) SENP3 truncates were illustrated (top). HEK293T cells were co-transfected with HA-Ub and GFP-SENP3 full length (FL) or truncates for 48 h. Cells were treated with MG132 (10 μM) for the later 12 h and H2O2 (100 μM) for the last 1 h. Co-IP using anti-GFP and IB using anti-HA were performed to determine the ubiquitination of SENP3, and GFP-SENP3 was detected with anti-GFP (middle). The domains of SENP3 were identified (bottom). (B) Myc-CHIP was co-transfected into HEK293T cells with FL or two truncates of GFP-SENP3 (N2 and C2) for 48 h, respectively. Cells were cultured in the presence of MG132 (10 μM) for the later 12 h. The proteins were co-immunoprecipitated using anti-Myc and immunoblotted using the indicated antibodies. (C) RGS-SENP3 was co-transfected into HEK293T cells with FL or two domains of Myc-CHIP (TPR and Ubox) for 48 h, respectively. Cells were cultured in the presence of MG132 (10 μM) for the later 12 h. The proteins were co-immunoprecipitated using anti-RGS and immunoblotted using the indicated antibodies. (D) HEK293T cells were transfected with GFP-SENP3 FL and truncates (N1, N2, N3, C1, C2, or C3), respectively, for 48 h. H2O2 was added for the last 1 h. Co-IP using anti-Hsp90 and IB using the indicated antibodies were performed to determine the binding of endogenous Hsp90 with SENP3 and its truncates. (E) A speculative model depicting the interplay among SENP3, Hsp90, and CHIP upon oxidative stress.