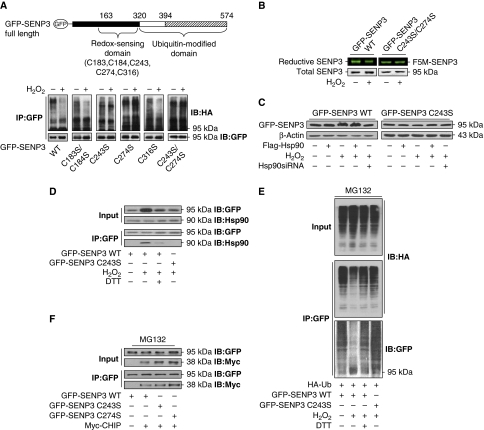
Figure 6.
Blockade of SENP3 ubiquitination is triggered by oxidative modification of cysteines on SENP3, which recruits Hsp90. (A) The identified domains of SENP3 and the sites for mutagenesis with cysteines replaced by serines in the redox-sensing domain (upper). HEK293T cells were co-transfected with HA-Ub and GFP-SENP3 wild-type (WT) or C/S mutants for 48 h. Cells were treated with MG132 (10 μM) for the later 12 h and H2O2 (100 μM) for the last 1 h. Co-IP using anti-GFP and IB using the anti-HA were performed to determine the ubiquitination of SENP3, and GFP-SENP3 was detected with anti-GFP (bottom). (B) HEK293T cells were transfected with GFP-SENP3 WT or C243/274S mutant for 48 h. Cells were treated with H2O2 (100 μM) for 30 min before incubation with F5M for another 30 min. IP with anti-GFP was performed. Total GFP-SENP3 protein levels were evaluated by IB with anti-GFP. Reductive GFP-SENP3 with F5M fluorescence in the same bands were visualized and photographed. (C) HEK293T cells were transfected with GFP-SENP3 WT or mutant C243S with or without Flag-Hsp90 or Hsp90 siRNA for 48 h, and 100 μM H2O2 was added for the last 1 h. SENP3 protein levels were evaluated by IB. (D) HEK293T cells were transfected with GFP-SENP3 WT or mutant C243S for 48 h. A measure of 100 μM H2O2 and 5 mM DTT were added for the last 1 h. The proteins were co-immunoprecipitated using anti-GFP and immunoblotted using the indicated antibodies to determine the binding of endogenous Hsp90 with WT or mutant SENP3. (E) HEK293T cells were co-transfected with HA-Ub and GFP-SENP3 WT or mutant C243S for 48 h and MG132 was added for the later 12 h. H2O2 and DTT were added for the last 1 h. The proteins were co-immunoprecipitated using anti-GFP and immunoblotted using the indicated antibodies to determine the ubiquitination of SENP3. (F) HEK293T cells were co-transfected with GFP-SENP3 WT or mutants C243S and C274S, and Myc-CHIP for 48 h. MG132 was added for the later 12 h. The proteins were co-immunoprecipitated using anti-GFP and immunoblotted using the indicated antibodies to determine the binding of Myc-CHIP with SENP3 WT or mutants.