Figure 4. Functional impact of Plexin mutations found in cancers.
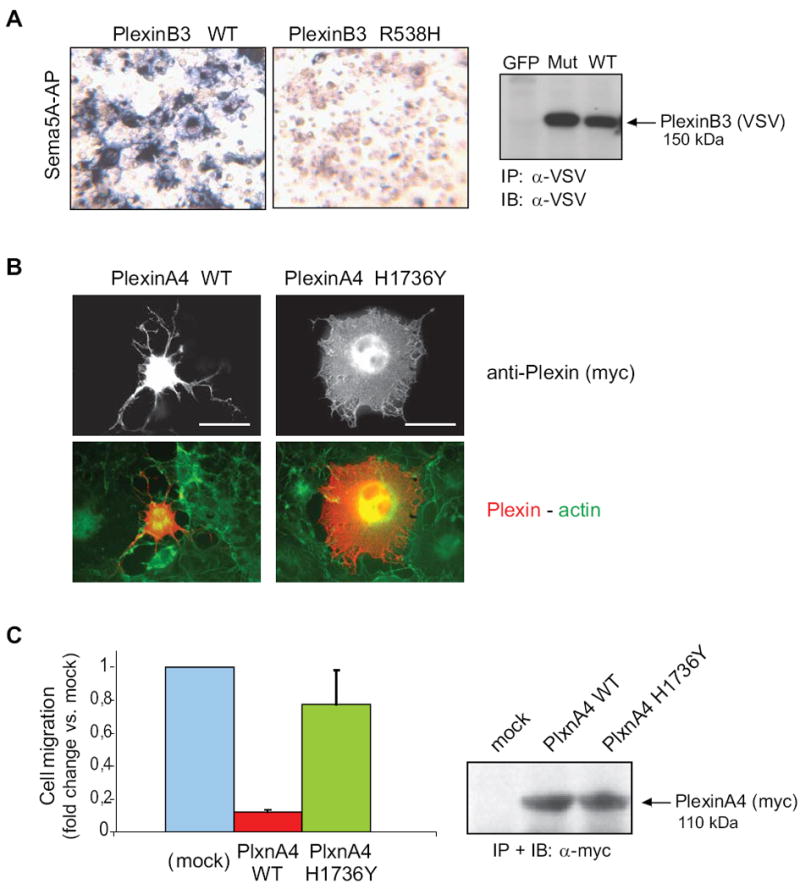
A, the functional impact of mutation p.R538H in the extracellular domain of PLXNB3 was assessed in COS cells (see Supplementary Methods for details) by challenging the receptor with a soluble form of the specific ligand Sema5A fused to alkaline phosphatase (Sema5A-AP), as previously shown (Artigiani et al., 2004). The comparable expression of the receptor proteins in the cells used for binding assays was confirmed by Western blotting (on the right). The results shown are representative of three independent experiments. The mutated extracellular domain of PLXNB3 has lost the ability to interact with Sema5A. B, the collapsing activity of either wild type or p.H1736Y mutated PLXNA4 was tested in COS cells, by expressing a constitutively active form of the receptor (see Methods), followed by immunofluorescence analysis with anti-Myc-tag antibodies (revealing the plexin) and phalloidin-FITC cell counterstaining (see merged images, at the bottom). Scale bar = 30 μm. The comparable expression of the two proteins was confirmed by Western blotting (on the right). Over 60% of the cells expressing wild-type active PlexinA4 displayed a collapsed phenotype (as shown in representative field), while the mutated plexin had lost this ability (yielding less then 10% collapsed cells). The results shown are representative of three independent experiments. C, wild type and p.H1736 mutated PLXNA4 (as in B) were further expressed in MDA-MB435 human melanoma cells, to investigate the impact of the mutation on the ability of plexin signals to inhibit tumor cell migration (see Methods for experimental details). Spontaneous cell migration was significantly inhibited in cells expressing the wild type activated form of PLXNA4, as expected, whereas cells expressing the mutated receptor were almost unaffected, indicating a functional loss. Protein expression levels were comparable (shown on the right).
