Abstract
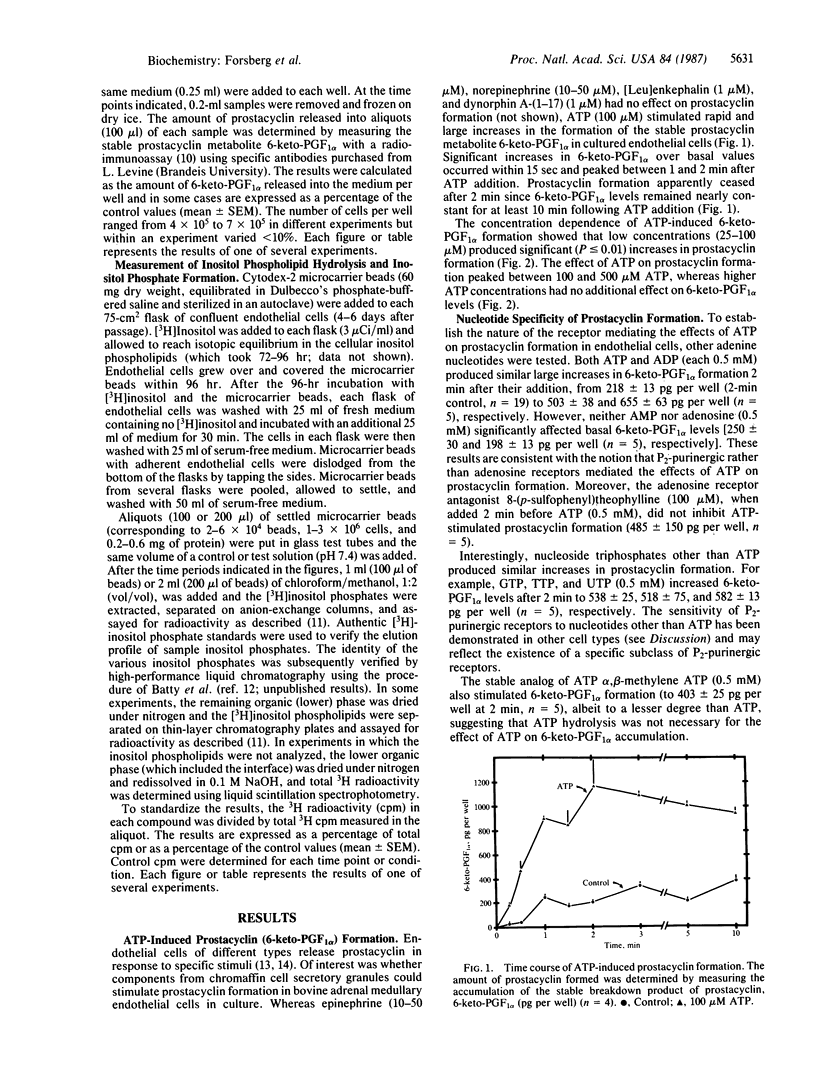
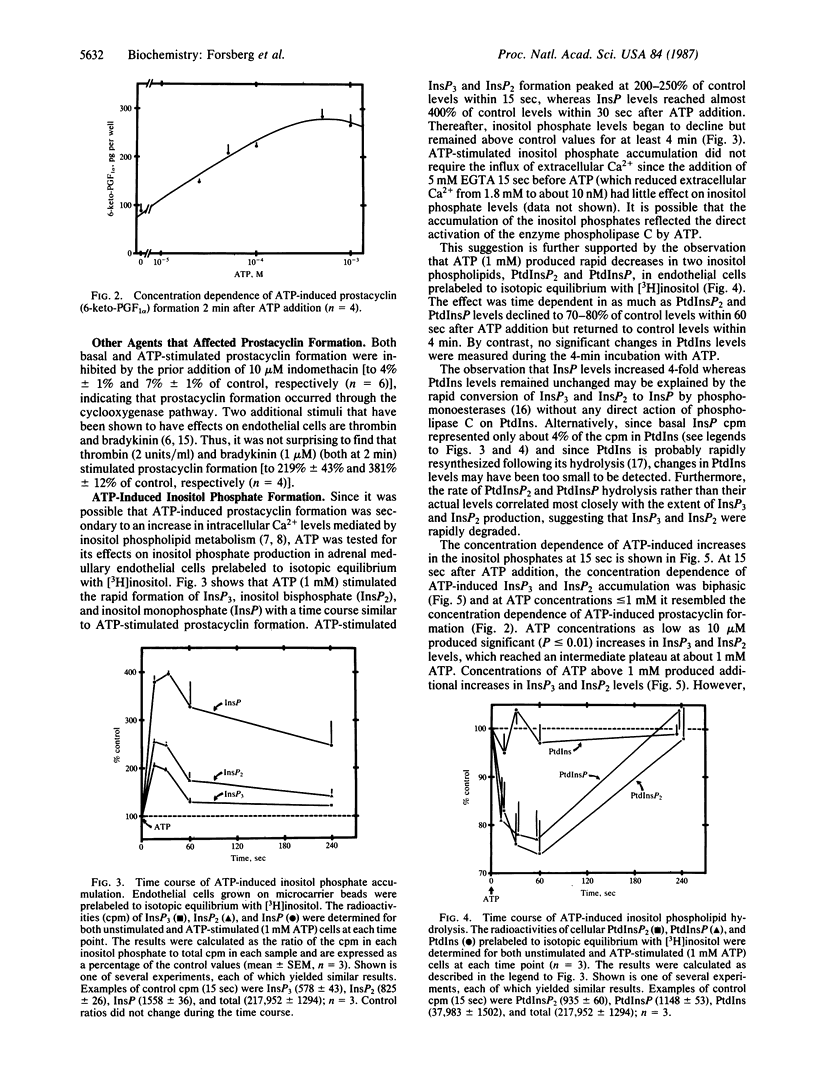
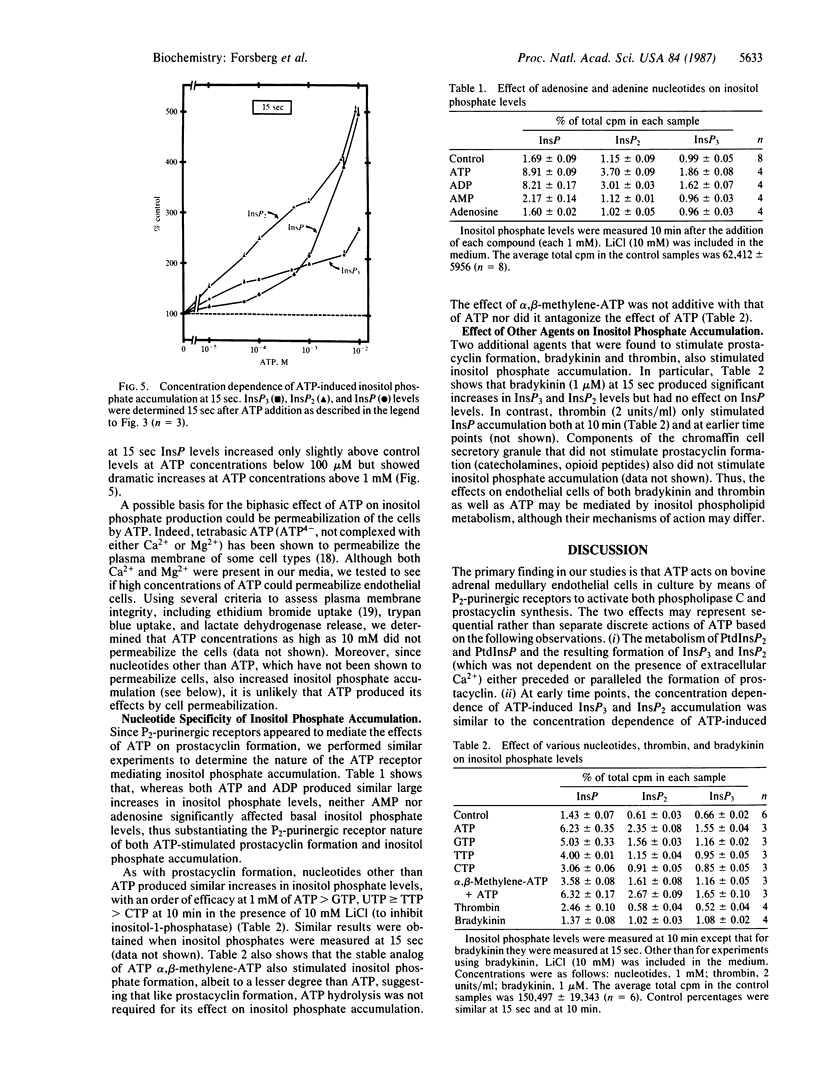
In the adrenal medulla, chromaffin cells secrete high concentrations of catecholamines, ATP, peptides and other factors that must pass through an endothelial cell barrier to enter the bloodstream. We have measured the effect of several of these chromaffin cell secretory products on cultured bovine adrenal medullary endothelial cells and have found that only ATP stimulates prostacyclin formation. The stimulation of prostacyclin formation by ATP coincides with the metabolism of inositol phospholipids and the accumulation of the putative second messenger inositol trisphosphate. The time course, concentration dependence, and P2-purinergic receptor specificity were similar for ATP-stimulated prostacyclin formation and ATP-stimulated inositol phospholipid metabolism. Thus, the increase in prostacyclin formation may be secondary to mobilization of intracellular Ca2+ by inositol trisphosphate, leading to activation of phospholipase A2, liberation of arachidonic acid, and the conversion of arachidonic acid to prostacyclin. We propose that the function of ATP, which is often colocalized with cell-specific hormones in secretory cells, may be to regulate blood flow in the adrenal medulla and other endocrine tissues by interacting with adjacent endothelial cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee D. K., Ornberg R. L., Youdim M. B., Heldman E., Pollard H. B. Endothelial cells from bovine adrenal medulla develop capillary-like growth patterns in culture. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4702–4706. doi: 10.1073/pnas.82.14.4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batty I. R., Nahorski S. R., Irvine R. F. Rapid formation of inositol 1,3,4,5-tetrakisphosphate following muscarinic receptor stimulation of rat cerebral cortical slices. Biochem J. 1985 Nov 15;232(1):211–215. doi: 10.1042/bj2320211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R. L., Kennerly D. A., Stanford N., Majerus P. W. Diglyceride lipase: a pathway for arachidonate release from human platelets. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3238–3241. doi: 10.1073/pnas.76.7.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. M., Burnstock G. The structural conformation of the polyphosphate chain of the ATP molecule is critical for its promotion of prostaglandin biosynthesis. Eur J Pharmacol. 1981 Jan 5;69(1):81–86. doi: 10.1016/0014-2999(81)90604-x. [DOI] [PubMed] [Google Scholar]
- Derian C. K., Moskowitz M. A. Polyphosphoinositide hydrolysis in endothelial cells and carotid artery segments. Bradykinin-2 receptor stimulation is calcium-independent. J Biol Chem. 1986 Mar 15;261(8):3831–3837. [PubMed] [Google Scholar]
- Forsberg E. J., Rojas E., Pollard H. B. Muscarinic receptor enhancement of nicotine-induced catecholamine secretion may be mediated by phosphoinositide metabolism in bovine adrenal chromaffin cells. J Biol Chem. 1986 Apr 15;261(11):4915–4920. [PubMed] [Google Scholar]
- Furchgott R. F. The role of endothelium in the responses of vascular smooth muscle to drugs. Annu Rev Pharmacol Toxicol. 1984;24:175–197. doi: 10.1146/annurev.pa.24.040184.001135. [DOI] [PubMed] [Google Scholar]
- Gaudet R. J., Alam I., Levine L. Accumulation of cyclooxygenase products of arachidonic acid metabolism in gerbil brain during reperfusion after bilateral common carotid artery occlusion. J Neurochem. 1980 Sep;35(3):653–658. doi: 10.1111/j.1471-4159.1980.tb03704.x. [DOI] [PubMed] [Google Scholar]
- Gomperts B. D. Involvement of guanine nucleotide-binding protein in the gating of Ca2+ by receptors. Nature. 1983 Nov 3;306(5938):64–66. doi: 10.1038/306064a0. [DOI] [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins P. T., Michell R. H., Kirk C. J. Analysis of the metabolic turnover of the individual phosphate groups of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate. Validation of novel analytical techniques by using 32P-labelled lipids from erythrocytes. Biochem J. 1984 Mar 15;218(3):785–793. doi: 10.1042/bj2180785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokin L. E. Receptors and phosphoinositide-generated second messengers. Annu Rev Biochem. 1985;54:205–235. doi: 10.1146/annurev.bi.54.070185.001225. [DOI] [PubMed] [Google Scholar]
- Kikuta A., Murakami T. Relationship between chromaffin cells and blood vessels in the rat adrenal medulla: a transmission electron microscopic study combined with blood vessel reconstructions. Am J Anat. 1984 May;170(1):73–81. doi: 10.1002/aja.1001700106. [DOI] [PubMed] [Google Scholar]
- Lapetina E. G., Billah M. M., Cuatrecasas P. The phosphatidylinositol cycle and the regulation of arachidonic acid production. Nature. 1981 Jul 23;292(5821):367–369. doi: 10.1038/292367a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W., Cusack N. J., Carleton J. S., Gordon J. L. Specificity of P2-purinoceptor that mediates endothelium-dependent relaxation of the pig aorta. Eur J Pharmacol. 1985 Feb 5;108(3):295–299. doi: 10.1016/0014-2999(85)90452-2. [DOI] [PubMed] [Google Scholar]
- Moncada S., Herman A. G., Higgs E. A., Vane J. R. Differential formation of prostacyclin (PGX or PGI2) by layers of the arterial wall. An explanation for the anti-thrombotic properties of vascular endothelium. Thromb Res. 1977 Sep;11(3):323–344. doi: 10.1016/0049-3848(77)90185-2. [DOI] [PubMed] [Google Scholar]
- Pollard H. B., Pazoles C. J., Creutz C. E., Scott J. H., Zinder O., Hotchkiss A. An osmotic mechanism for exocytosis from dissociated chromaffin cells. J Biol Chem. 1984 Jan 25;259(2):1114–1121. [PubMed] [Google Scholar]
- Rittenhouse-Simmons S. Production of diglyceride from phosphatidylinositol in activated human platelets. J Clin Invest. 1979 Apr;63(4):580–587. doi: 10.1172/JCI109339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas E., Pollard H. B., Heldman E. Real-time measurements of acetylcholine-induced release of ATP from bovine medullary chromaffin cells. FEBS Lett. 1985 Jun 17;185(2):323–327. doi: 10.1016/0014-5793(85)80931-5. [DOI] [PubMed] [Google Scholar]
- Schwartzman M., Pinkas R., Raz A. Evidence for different purinergic receptors for ATP and ADP in rabbit kidney and heart. Eur J Pharmacol. 1981 Sep 11;74(2-3):167–173. doi: 10.1016/0014-2999(81)90527-6. [DOI] [PubMed] [Google Scholar]
- Smith W. L. Prostaglandin biosynthesis and its compartmentation in vascular smooth muscle and endothelial cells. Annu Rev Physiol. 1986;48:251–262. doi: 10.1146/annurev.ph.48.030186.001343. [DOI] [PubMed] [Google Scholar]
- Storey D. J., Shears S. B., Kirk C. J., Michell R. H. Stepwise enzymatic dephosphorylation of inositol 1,4,5-trisphosphate to inositol in liver. Nature. 1984 Nov 22;312(5992):374–376. doi: 10.1038/312374a0. [DOI] [PubMed] [Google Scholar]
- Ungar A., Phillips J. H. Regulation of the adrenal medulla. Physiol Rev. 1983 Jul;63(3):787–843. doi: 10.1152/physrev.1983.63.3.787. [DOI] [PubMed] [Google Scholar]
- Winkler H., Westhead E. The molecular organization of adrenal chromaffin granules. Neuroscience. 1980;5(11):1803–1823. doi: 10.1016/0306-4522(80)90031-7. [DOI] [PubMed] [Google Scholar]
- Youdim M. B., Banerjee D. K., Pollard H. B. Isolated chromaffin cells from adrenal medulla contain primarily monoamine oxidase B. Science. 1984 May 11;224(4649):619–621. doi: 10.1126/science.6424235. [DOI] [PubMed] [Google Scholar]


