Abstract
Only a few reports exist concerning biomechanical challenges spine surgeons face when treating Parkinson’s disease (PD) patients with spinal deformity. We recognized patients suffering from spinal deformity aggravated by the burden of PD to stress the principles of sagittal balance in surgical treatment. Treatment of sagittal imbalance in PD is difficult due to brittle bone and (the neuromuscular disorder) with postural dysfunction. We performed a retrospective review of 23 PD patients treated surgically for spinal disorders. Mean ASA score was 2.3 (2–3). Outcome analysis included review of medical records focusing on failure characteristics, complications, and radiographic analysis of balance parameters to characterize special risk factors or precautions to be considered in PD patients. The sample included 15 female and 8 male PD patients with mean age of 66.3 years (57–76) at index surgery and 67.9 years (59–76) at follow-up. 10 patients (43.5%) presented with the sequels of failed previous surgery. 18 patients (78.3%) underwent multilevel fusion (C3 level) with 16 patients (69.6%) having fusion to S1, S2 or the Ilium. At a mean follow-up of 14.5 months (1–59) we noted medical complications in 7 patients (30.4%) and surgical complications in 12 patients (52.2%). C7-sagittal center vertical line was 12.2 cm (8–57) preoperatively, 6.9 cm postoperatively, and 7.6 cm at follow-up. Detailed analysis of radiographs, sagittal spinal, and spino-pelvic balance, stressed a positive C7 off-set of 10 cm on average in 25% of patients at follow-up requiring revision surgery in 4 of them. Statistical analysis revealed that patients with a postoperative or follow-up sagittal imbalance (C7-SVL >10 cm) had a significantly increased rate of revision done or scheduled (p = 0.03). Patients with revision surgery as index procedure also were found more likely to suffer postoperative or final sagittal imbalance (C7-SPL, 10 cm; p = 0.008). At all, 33% of patients had any early or late revision performed. Nevertheless, 78% of patients were satisfied or very satisfied with their clinical outcome, while 22% were either not satisfied or uncertain regarding their outcome. The surgical history of PD patients treated for spinal disorders and the reasons necessitating redo surgery for recalcitrant global sagittal imbalance in our sample stressed the mainstays of spinal surgery in Parkinson’s: If spinal surgery is indicated, the reconstruction of spino-pelvic balance with focus on lumbar lordosis and global sagittal alignment is required.
Keywords: Parkinson’s disease, Spine surgery, Adult spinal deformity, Sagittal balance, Failure, Spino-pelvic
Introduction
In spinal deformity surgery, distinct syndromatic disorders, such as osteogenesis imperfecta, Marfan and Ehlers–Danlos Syndrome, fibrous dysplasia or neurofibromatosis have been found to imply increased risks and complications, which need special pre- and postoperative consideration. In the authors’ experience, patients with Parkinson’s disease (PD) also resemble a patient subgroup where the underlying medical disease poses significant challenges for the treatment of spinal pathologies, in particular for the reconstruction of a physiological sagittal profile and spinal balance.
The importance of spino-pelvic balance as a function of spinal and pelvic regulators yielding for the adjustment of physiological sagittal alignment in healthy volunteers as well as in patients with spondylolisthesis, scoliosis, kyphosis or degenerative lumbar disease has been shown [3, 8, 18–20, 29–31, 35, 38, 43]. Likewise, the impact of a physiological spino-pelvic alignment on clinical outcomes has been demonstrated repeatedly, and sagittal imbalance has been shown to cause worse outcomes following spinal surgery [3, 5, 15, 16, 20, 25, 26, 28, 30, 33, 38, 42].
In PD, as a chronic neurodegenerative disorder of the basal ganglia, posture is often affected because the postural reflexes necessary for upright stance and walk are disturbed. Postural instability, which increases with duration and severity of the disease including gait disorders, balance impairments with subsequent falls and fall-related injuries affects the quality of life negatively [4, 7]. The cardinal features of PD are rest tremor, rigidity and bradykinesia; disease-specific features include stooped posture, slow movements, trunk imbalance with shuffling gait, and expressionless facies [4]. To spine surgeons, PD usually presents as a neuromuscular disorder in elderly patients displaying slight to severe postural impairment posed on a significant spinal deformity or degenerative instability with stenosis [4]. Inadequate data concerning spinal surgery in patients with PD exist, which report of high complication rates, including multiple reoperations in 86% and construct failure in 29% of patients [2].
The purpose of this study was therefore to identify disease-specific characteristics and risk factors that might help physicians treating patients with PD and spinal disorders.
Materials and methods
An international 2-center retrospective review study was performed. One of the authors (J.Z.) analyzed the institutional database, including more than 10,200 cases covering a 9-year period at a European spine center. The other author (F.A.) reviewed an operative record archive at an adult spinal deformity practice in the US. Inclusion criteria were patients with PD and spinal disorders of the thoracic or lumbar spine that were treated with instrumented spinal fusion. Acute trauma and tumor patients were excluded. Medical records were studied in detail. ASA classification, number and details of revision surgeries, major medical and any surgical complication such as deep wound infection, pseudoarthrosis, junctional kyphosis or construct failure was documented. Complications were defined as early if they occured within ≤3 months. All patients’ courses were analyzed and assessment of follow-up was done according to identical protocols at 6 months, 1, 2, and 5 years postoperatively, including biplanar full-standing radiographs and patient self-rated satisfaction. Biplanar preoperative, postoperative and follow-up full-standing radiographs were analyzed for standard spinal and spino-pelvic parameters [18]. Changes in construct alignment, failure of instrumentation, and evidence of pseudoarthrosis were noted. Adjacent-level decompensation was classified into osteoporotic insufficiency fractures and adjacent segment instability (transition syndrome/segmental collapse). We analyzed the proximal junction sagittal Cobb angle (PJA) according to Bridwell [26]. Proximal junctional kyphosis (PJK) was defined as a PJA >10°. Diagnosis of pseudoarthrosis was made when there was radiographic evidence of instrumentation failure, dislodgement or loosening, motion during surgical exploration, frank non-union on plain radiographs or using CT-scans. For statistical analysis, patients were differentiated whether they had had revision surgery or if they were scheduled for revision surgery due to construct failure, pseudoarthrosis or recurrence of sagittal imbalance.
23 consecutive patients were identified retrospectively. The sample included 8 male and 15 female patients with a mean age of 66 years. 18 patients had mid- to long-term data (MTL-data) available with >6 months of follow-up and full radiographic series and records available. 5 patients had either only short-term follow-up or their radiographic or clinical follow-up was incomplete because only copies of biplanar radiographs were sent for our consultation to save pains with traveling. Nevertheless, because of the sparse data on patients with PD, we included all patients.
10 patients (43.5%) were referred for failed previous surgery and were scheduled for revision surgery as index procedure. Mean number of previous surgeries was 0.9 ± 1.1 per patient (range, 0–4). 10 patients (43.5%) showed a scoliotic thoracic or lumbar curve at time of index procedure. This frequently added to the clinical picture known as ‘Pisa syndrome’ in patients with PD (Fig. 1).
Fig. 1.
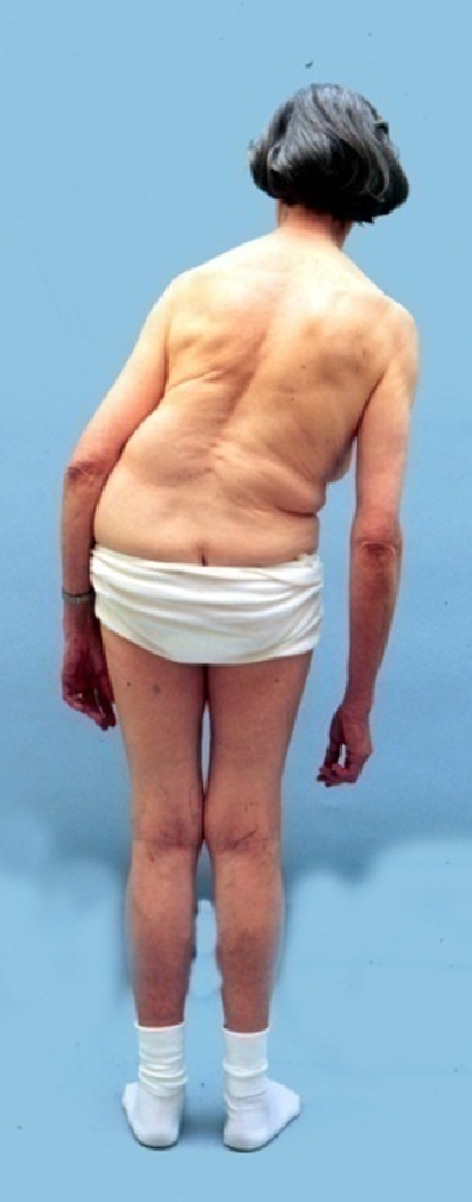
Pisa syndrome. Patient displays advanced right-sided lateralization with trunk-shift to the right due to the neuromuscular imbalance, here imposing on a right-sided convex degenerative lumbar scoliosis
18 patients (78.3%) presented at index surgery with global sagittal imbalance that was defined as the C7-sagittal center vertical line (C7-SVL) >3 cm anterior to the S1-posterior corner. A second tidemark was >10 cm anterior to the S1-posterior corner [18] identifying patients with the plumb line anterior to the hip axis resembling a decompensated state of sagittal imbalance.
Except for 5 patients, all had at least one additional co-morbidity indicating medical treatment beside the PD while the ASA score averaged 2.3 ± 0.4 points (range, 2–3 points).
The patients’ diagnosis included ‘degenerative lumbar instability with/without stenosis or spondylolisthesis’ (10×), degenerative scoliosis (9×), posttraumatic kyphosis at a single or multiple levels (6×), spondylodiscitis (2×) and postoperative wound infection (3×) including combinations thereof. In summary, most patients had significant morbidity indicating multilevel deformity surgery. Accordingly, surgical treatment was performed for decompression, reconstruction of sagittal alignment, and solid fusion using top-loading pedicle screw-constructs in all patients. Patients with PD do have brittle, osteopenic bone. If sagittal plane correction and/or lumbosacral fusion were indicated, anterior release and fusion were performed to reduce corrective forces with subsequent instrumented posterior correction. Anterior open transthoracic or thoracoabdominal approaches were performed in 13 patients (56.5%). Corpectomies were performed in 4 patients (17.4%). One patient had a L4 pedicle substraction osteotomy. Smith-Peterson type osteotomies or transversotomies were frequently applied within the posterior release, in particular in patients with prior surgeries. 1 patient suffered from global kyphosis after insufficient multilevel vertebroplasties indicating halo-gravity traction, multilevel anterior partial corpectomies, an anterior reconstruction using autologous fibula, and posterior instrumented fusion T10–L4. Autogenous bone was used in all patients except one who received bone substitutes.
18 patients had multilevel surgery at index surgery with a mean of 6.1 ± 4.3 levels (range, 1–16) included in the instrumented fusion. There was a total of 19 patients (82.6%) that had fusion to the sacrum. 4 of these patients (17.4%) had long fusions augmented with additional S2-screws and 3 (16%) with ilium screw fixation.
All patients were invited for follow-up, 82.6% of patients succeeded to present for clinical and radiographic follow-up after 4–6 months according to institutional protocol. The other patients had further clinical follow-up at their physician’s office and several patients with PD could not complete regular clinical and radiographic follow-up after 1, 2, and 5 years, as scheduled, due to their underlying disease, age, and co-morbidities.
Patients’ perioperative variables are summarized in Table 1. Including demographic variables, radiographic and binary outcome parameters (factor levels: yes/no) we yielded for a risk factor identification for ‘revision surgery done/scheduled for construct failure, pseudoarthrosis or sagittal imbalance’. For doing so, cross-tabulation tables together with two-tailed Fisher’s Exact tests were computed and evaluated. For all analyses, Statistica 6.1 (StatSoft, Tulsa/US) was used. A p value less than 5% indicated statistical significance.
Table 1.
Patients’ demographics and preoperative parameters
| Sample characteristics | Mean |
|---|---|
| Gender ratio female:male | 15:8 |
| ASA score | 2.3 |
| Age at index surgery | 66 years |
| Age at follow-up | 68 years |
| Follow-up | 14.2 months |
| Failed previous surgery | 43.5% |
| Global sagittal imbalance | 78.3% |
| Scoliosis | 43.5% |
Results
Patients’ demographics and results are summarized in Tables 1 and 2. The sample of 23 patients had a mean age at index surgery of 66.3 ± 5.9 years (range, 57–76). Mean age at follow-up was 67.9 ± 5.8 years (range, 59–76). At follow-up, which was 14.3 ± 14.3 months (range, 1–59), 11 patients were satisfied with the clinical outcome, 6 patients were very satisfied, 2 patients were neither satisfied nor dissatisfied, and 2 were not satisfied. In 2 patients, clinical outcome data were not available. At all, 14 patients (78%) with MTL-data were satisfied or very satisfied with clinical outcome while 4 patients (22%) were either not satisfied or uncertain regarding their outcome. 7 patients (30.4%) had documented medical complications during the perioperative course including appendicitis indicating surgery (1×), postoperative delirium (3×), liver decompensation with temporary hepatic encephalopathia indicating intensive care (1×), pneumothorax after central venous catheterization (1×), akinetic crisis indicating intensive neurologic care (1×), decompensation of diabetes mellitus type I (1×), and decompensation of kidney insufficiency (1×). No patient died during clinical stay or suffered any neurovascular complication. 12 patients (52.2%) had surgical complications including 3 patients with deep and 2 with superficial wound infection indicating revision surgery in 3 patients. One patient was found to suffer a lymphocele following anterior lumbar fusion that spontaneously stopped draining. 1 slender patient had a prominent symptomatic S2-alar screw indicating removal during a pseudarthrosis repair at a distant level. Finally, 7 patients (38.9% of patients with MTL-data) developed a pseudarthrosis after index procedure, all at the lumbosacral junction.
Table 2.
Summary of main results
| Clinical outcomes | % | N |
|---|---|---|
| Medical complications | 30.4 | 7 |
| Surgical complications | 52.2 | 12 |
| Adjacent segment collapse/insufficiency fractures | 17.6a | 3 |
| Proximal junctional kyphosis | 17.6a | 3 |
| Fusion after index procedure | 58.8a | 10 |
| Any early perioperative or late revision (included scheduled) | 35 | 8 |
| Fusion to S1, S2 or ilium | 69.6 | 16 |
| Multilevel fusion (>3 levels) | 78.3 | 18 |
| Levels fused | – | 6.3 |
| Patients satisfied/very satisfied | 88a | 15 |
a3/17 pat with mid- to long-term data available
In summary, 17% of patients had infection-related and 35% of patients construct or fusion-related complications. Revision surgery was performed in 6 of 18 patients (33.3%) with MTL-data available. 2 others were indicated with one scheduled and one denying further interventions. 1 revision was done in 3 patients and 2 revisions were done in 3 patients. Summarizing any early postoperative or late revision surgery as well as indicated or scheduled surgeries for construct failure, pseudoarthrosis or sagittal imbalance, the total revision rate would be 34.7%. The cause of revisions performed, indicated or scheduled was infection in 3 patients and/or pseudoarthrosis with/without construct failure as well as recurrence of sagittal imbalance in the remaining 5 patients.
Analysis of radiographs was performed in all patients. Data of the radiographic course are summarized in Table 3. Notably, preoperatively those 18 patients subjected to multi-level instrumented spinal fusions revealed a mean pelvic tilt of 31° resembling a retroverted pelvic position compensating for a global kyphotic alignment with the C7 plumb line a mean of 13.7 cm anterior to the S1 postero-superior corner.
Table 3.
Radiographic results
| Coronal plane | Sagittal plane | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C7-CVL (cm) | Thoracic curve (deg) | Lumbar curve (deg) | Kyphosis T4–T12 (deg) | Lordosis L1–S1 (deg) | C7-SVL (cm) | Sacral slope (deg) | Pelvic tilt (deg) | Pelvic incidence (deg) | |
| Preoperative | 2.7 ± 3.5 (0–15) | 8.3 ± 7.9 (0–25) | 14.6 ± 14.0 (0–43) | 30.5 ± 15.8 (−12 to 50) | −8.8 ± 22.3 (5 to −86) | 12.2 ± 10.9 (−1.5 to 39 ) | 33.9 ± 13.1 (8–57) | 31.7 ± 8.2 (21–50) | 61.3 ± 15.6 (42–88) |
| Postoperative | 1.5 ± 2.3 (0–8) | 5.0 ± 5.2 (0–16.8) | 2.9 ± 3.4 (0–10) | 32.7 ± 12.1 (10–48.6) | −46.0 ± 12.6 (−26 to −74) | 6.9 ± 5.8 (−0.5 to 20) | 35.0 ± 12.4 (15–65) | 23.2 ± 10.6 (8–45) | 54.1 ± 13.3 (33.6–75) |
| Follow-up | 1.9 ± 2.3 (1–7) | 6.0 ± 6.9 (0–18) | 5.7 ± 6.1 (0–17) | 35.7 ± 12.6 (10–53) | 46.3 ± 12.4 (17 to −66) | 7.2 ± 6.1 (−2.8 to 20) | 36.5 ± 10.4 (19.6–58) | 27.7 ± 10.7 (10–45) | 60 ± 14.9 (34–92) |
C7-CVL C7-coronal center vertical line, C7-SVL C7-sagittal center vertical line
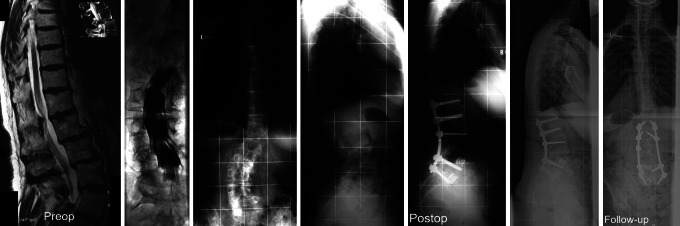
The radiographic spino-pelvic balance parameters were not subjected to statistical analysis of correlations in light of the comprehensive sample size and the fact that several patients had obesity rendering morphometric measurements around the hips difficult (Fig. 2). At index surgery, 18 patients (78.3%) displayed global sagittal imbalance (C7-SVL >3 cm anterior to S1). In 9 patients (39%) the C7-SVL was >10 cm anterior to the S1-posterior corner resembling decompensated sagittal imbalance. On follow-up radiographs, 3 out of 18 patients (16.7%) with MTL-data had evidence of adjacent level instability with stenosis and kyphosis due to an osteoporotic insufficiency fracture. Also, 3 out of 18 patients (16.7%) with MTL-data had a PJK at follow-up. Analysis of radiographs resulted in an index union rate of 61.1% only (11 of 18 patients with MTL-data) that increased to 77.8% (14 of 18 patients) at latest follow-up. The others denied further surgery (2), are scheduled for revision surgery after weight loss (1) or were lost to follow-up (1). Notably, the only two patients that could be treated with a single-level decompression displayed albeit physiological alignment and had an instrumented fusion for a focal stenosis. These patients experienced an uneventful course.
Fig. 2.
45-year-old patient with long-lasting back pain nonresponsive to conservative treatment. ASA-classification was 3, PD lasted for more than 5 years. Radiographs displayed degenerative lumbar scoliosis with fixed sagittal imbalance and multiple osteoporotic compression fractures with significant lumbar stenosis at L3–L5. Treatment was with anterior release and fusion L1–S1 with corpectomies of L3 and L4 and second-stage posterior reconstruction of lordosis and spino-pelvic alignment and posterior fusion T12–S2 using iliac crest bone. Screws in S2 were used to augment the lumbopelvic fixation in the patient with osteoporotic bone
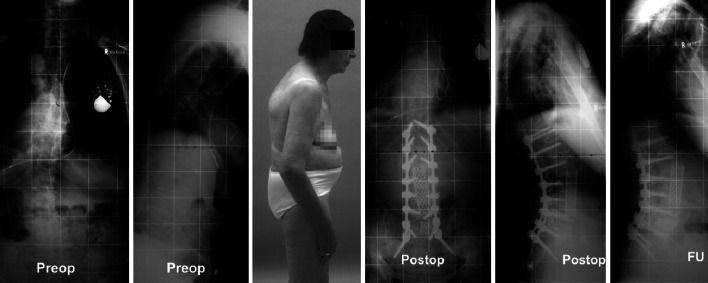
Global sagittal imbalance with the C7-SVL >10 cm in front of the S1 posterior corner or with a postsurgical loss of the corrected C7-SVL of >5 cm on full standing lateral radiographs was noted in 5 of 20 patients (25%) with MTL-data available. A positive C7-SVL >10 cm was observed in 5 of 20 patients (25%) with radiographic MTL-data indicating revision in 4 of them including 2 patients with PJK and 1 patient with adjacent segment collapse.
Statistical analysis revealed that patients with a postoperative or follow-up sagittal imbalance (C7-SVL >10 cm) had a significantly increased rate of revision surgery done or scheduled (p = 0.031). Patients with revision surgery as index procedure also were found more likely to suffer postoperative or final sagittal imbalance (C7-SVL >10 cm; p = 0.008). Patients with PJK were also likely to suffer global sagittal imbalance (p = 0.025) at final follow-up.
We noticed that incomplete correction of spino-pelvic alignment in PD patients caused recalcitrant sagittal imbalance due to the disease-related forward ‘pull’ of the trunk and gravity line while the degree of postural instability correlated with increasing severity of the neuromuscular disease. The latter can finally lead to clinical signs of camptocormia. Our observations are described in detail and illustrated in Fig. 3. Summarizing, although spinal and spino-pelvic parameters like lumbar lordosis were corrected in favor of a physiological alignment in the multilevel fusion group with a postoperative mean lumbar lordosis (Cobb L1–S1) of 45° ± 12.7° (range, 26°–74°) and pelvic tilt of 21.6° ± 8.9° (range, 8°–37°) in that group, some patients with late failure showed incomplete correction of the spino-pelvic alignment that contributed to final construct failure, pseudoarthrosis, and the need for revision surgery. However, probably due to the limited sample size, we could not prove this interdependency of failed lumbo-pelvic balance and ‘revision surgery done or scheduled’.
Fig. 3.
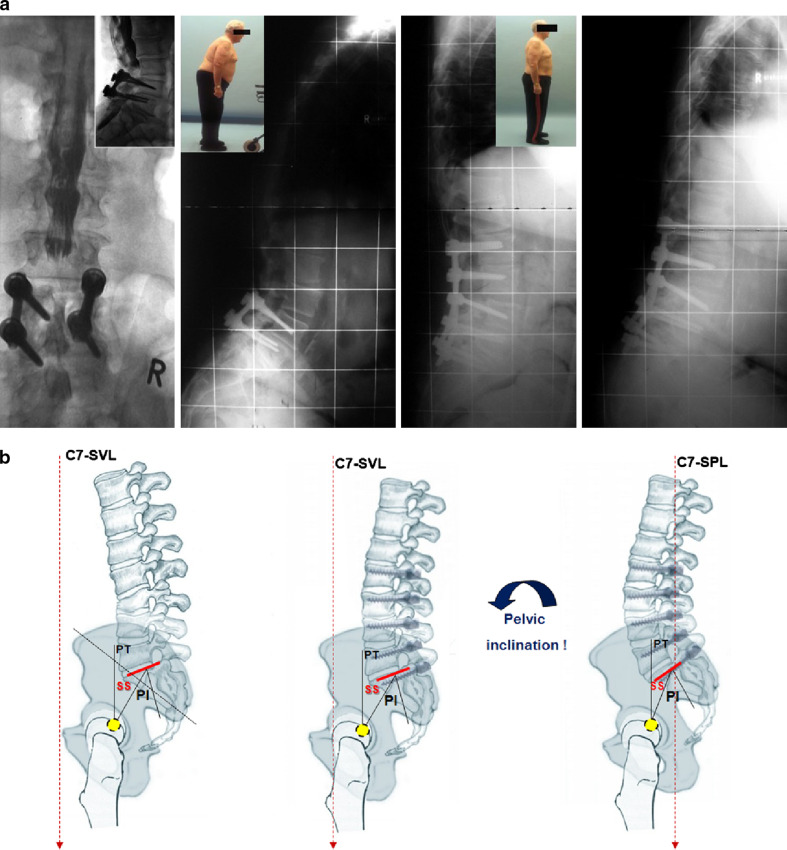
a Representative case example of PD patient with failed reconstruction of sagittal balance. Male patient, 66 years old, ASA-score 2, weight was 134 kg. The patient presented with a previous posterior instrumented spinal fusion L4–5, rod breakage and pseudoarthrosis with two subsequent revisions for wound infection. Our treatment was with irrigation and debridement, instrumented posterior revision fusion and decompression L3–S1. Postoperative reccurent wound infection indicated revision. Postoperative alignment showed reconstructed lumbar lordosis but pelvic tilt remained in compensatoric, retroverted position. Follow-up radiographs and clinical examination revealed sagittal imbalance due to pseudoarthrosis at L5–S1 with rod breakage as well as L2 adjacent-level compression fracture. Patient displayed significant muscle fatigue, advanced severity of PD further more contributing to the global spinal imbalance. The patient is scheduled for revision after weight loss. b Importance of complete spino-pelvic balance correction in patients with sagittal imbalance. Explanation of failure in a the patient experienced recurrent sagittal imbalance due to an incomplete correction of the spino-pelvic alignment. In the first revision surgery lumbar lordosis was corrected from kyphosis (left) to slight lumbar lordosis (middle). But, the pelvis was still in retroversion (high pelvic tilt, PT) indicating incomplete correction of spino-pelvic alignment that subsequently caused global, recalcitrant imbalance. Right figure illustrates correct alignment with a balanced spino-pelvic unit including harmonic lordosis and an inclined, normalized pelvic tilt
Discussion
Adults older than 50 years are projected to be the fastest growing segment of the adult population. Coincidentally, our increasing medical capacity to offer advanced spinal surgery, in particular, to elderly patients with significant co-morbidities, will increase the number of patients with PD seeking spinal care. Adult spinal deformity is a complex disorder that affects up to 60% of the aging population [36]. Adult spinal surgery goes with a significant rate of complications and revision surgery. A review of the literature regarding adult spinal deformity surgery, including studies with sample sizes of at least 40 patients, reveals an overall complication rate lingering around one-third of cases with revision and subsequent surgeries indicated in one-quarter of patients treated [9, 10, 13, 34, 40, 44]. Risk factors for failure were elucidated in a report on 144 patients by Kim et al. [27]: Besides a thoracolumbar kyphosis and osteoarthritis of the hip, positive postoperative sagittal balance of 5 cm or more, age >55 years, and inadequate sacropelvic fixation were shown as statistically significant risk factors for poor outcome. Postoperative sagittal imbalance was suggested to increase the tensile forces on the posterior implants and fusion mass subsequently promoting construct failure. These previous studies on adult spinal deformity surgery serve evidence that spine surgeons might have to expect a revision for complications or subsequent surgeries at or adjacent to the previous instrumentation in one-third of patients on average. Notably, as in the study of Mok [34], adults with neuromuscular diseases were frequently not included or underrepresented in published articles. Likewise, spine literature is reluctant offering sufficient information on adult deformity surgery in patients with neuromuscular diseases. There are neither guidelines for treatment nor sufficient information when physicians look for the distinct challenges to be expected in PD patients.
With a detailed analysis of PD patients undergoing spinal surgery we shared our experiences on 23 patients with PD being the largest cohort reported. Mean age was 66 years, comparable to cited studies on adult deformity surgery. When we compared the results derived by the current series, the early preoperative and late revision rate compares well with the published data mentioned above. However, in our experience the overall complication and revision rate was striking. In our database we identified an overall infection rate in spinal surgeries of below 1%; it was 17% in our current series on PD patients and 14% in a series of Babat [2, 18]. That author [2] reported on the elevated risk of spinal surgery in PD. In a 7-year period the authors identified 14 patients and reported the results of a 3-centers experience. The sample included 3 male and 11 female PD patients with mean age of 71 years and follow-up of 67 months. Similar to the current study 4 patients were lost to follow-up within 1 year after index surgery and MTL-data were available for 79%. 9 patients (64%) had co-morbidities. 4 surgeries were performed in the cervical spine and 10 in the thoracic or lumbar spine. 10 patients could be examined or interviewed at a mean follow-up of 40 months. 12 patients had a total of 31 reoperations and 11 patients (79%) underwent 22 revision procedures for instability or decompensation at the same or adjacent levels. 4 of these patients had pull-out or hardware failure a total of 10 times. Notably, all 5 patients that were treated with decompression alone developed instability at that level indicating revision with instrumented fusion. In addition, all of the 3 multilevel fusions and one single-level fusion required additional surgery. All failures resulted from progressive kyphosis at the operated or junctional levels. Summarizing, multiple operations were indicated in 86% of the sample with 2.6 surgeries per patient on average (range, 1–11).
Babat concluded in 2004 that the combination of a serious neuromuscular disorder and poor bone stock exposes these patients to elevated risks and repeated surgeries. The common mechanism of failure was assumed to be the kyphosis adjacent to the levels operated on initially. Actually, PD was shown to confer poor bone quality [21–23] and our observations echo those of Babat. Poor fusion-level selection, neglect, and a lack of reconstruction of sagittal balance are frequent mistakes that can cause failure of the index surgery [14, 15, 26, 30, 37], but, failure of spinal surgery in PD patient does have additional risk factors that are difficult to control.
With increasing disease durance and severity, some patients presenting with PD display advanced trunk imbalance and inclination, also referred to as ‘camptocormia’ (Fig. 3) as well as marked lateral trunk shift, also referred to as ‘Pisa syndrome’ (Fig. 1) [4]. Camptocormia is an abnormal posture with marked flexion of the thoracolumbar spine, and it increases with time and fatigue during the day and during walking; it is a progressive postural insufficiency that abates in recumbent position, sitting or volitionally when the PD patient leans against a wall. There are usually no radiographic abnormalities or structural changes referring to that kind of neuromuscular dysfunction [1, 12, 24, 32, 39]. Camptocormia in PD patients was observed with an incidence of 7% dependant on the clinical severity of the PD. Previous spinal surgery was found a risk factor for the onset of camptocormia and we noted that surgical efforts for sagittal plane reconstruction as well as the postoperative maintenance of upright stance and walk are particularly difficult in this subgroup of patients. This kind of global kyphosis is difficult to address surgically. The flexed posture during stance and walk adds significant bending stress on any instrumented fusion (Fig. 3).
Recommendations
PD patients are a high-risk population in adult spinal surgery. With our results and that of Babat in mind we might formulate recommendations at the level of experts’ opinion, for the treatment of PD patients with spinal disorders.
Surgeons considering treatment of patients with PD should anticipate increased medical and surgical risks and need experience with adult deformity surgery and the demands of revision surgeries. Accordingly, nonsurgical therapy should be maxed out.
Osteoarthritis of the hip can aggravate the lack of existing spinal and spino-pelvic compensatory mechanisms. If surgery is indicated, osteoarthritis of the hip should be ruled out and if symptomatic, addressed prior to spinal surgery.
PD patients with maintained sagittal balance or with compensated sagittal imbalance (C7-SVL >5 cm but behind hip axis) and a focal spinal problem, such as 1-level stenosis, should be evaluated for an instrumented fusion added to any decompression procedure. Being aware of the sparse evidence supporting fusion added to decompression surgery in PD patients, failure with non-instrumented surgery as reported by Babat and the successful fusion-cases in our series might encourage the addition of an instrumented fusion.
A primary goal in surgical treatment of adult spinal deformity is to achieve proper alignment for ergonomic standing [11]. To succeed, the surgeon must take into account not only the global spinal balance but also the pelvic position [30] (Fig. 4). Likewise, in PD patients who have multilevel spinal disease and/or decompensated sagittal imbalance, a thorough analysis of the sagittal and coronal plane should be done. PD patients frequently have scoliosis [17], 43.5% in the current series, and thus trunk imbalance in the coronal plane has to be taken into surgical calculations as well. In order to maintain the gravity line balanced, patients with spinal disorders in the sagittal plane can recruit balancing mechanism such as pelvic retroversion in case of loss of lumbar lordosis or adjustments in thoracic kyphosis and at adjacent-levels to a spinal deformity, fusion mass or degenerated spinal segment. In PD patients the muscular tension band is weak, prone to fatigue and spinal adjustment with compensation adjacent to a surgical fusion that did not achieve physiological contour is unlikely. Therefore, reconstruction of physiological lordosis and lumbo-pelvic parameters is crucial when considering multi-level fusions even though this may indicate fusion into the thoracic spine.
Fig. 4.
60-year-old patient with severe back pain and adult degenerative left convex scoliosis. 2-year history of medical treatment for PD. Preop diagnostics displayed maximum stenosis and degenerative instability at L1–2. Treatment was with instrumented posterior scoliosis correction and fusion L1–S1 and second stage anterior fusion L4–S1 using titanium mesh cages and iliac crest bone. Outcome at 7 months follow-up showed solid union and maintenance of the lordotic alignment L1–S1. However, comparison of postoperative and follow-up radiographs revealed an anterior shift of the upper trunk with clinical image of worsened sagittal posture. The patient showed advanced signs of the Parkinson’s posture since the last visit at our institution and was recommended for closer surveillance by her neurologist
As PD patients do have a weak muscular posterior tension band, a generally flexed and stooped posture increases with disease severity and unfavorable biomechanics of a long lever arm at the lumbosacral junction result. This poses large forces on lumbosacral instrumentations. Hence, the threshold for using additional sacral fixation points (S2-screws [6]) or iliac screw fixation [41] should be low which can increase fusion rates at L5–S1 in high-risk non-union patients [40].
Conclusion
Parkinson’s is a debilitating disease that typically occurs in an older population; thus, patients often present with a combination of bridle bone, postural dysfunction, and known risks for medical as well as surgical complications of adult spinal surgery. Surgical treatment is troublesome due to the biomechanical challenges posed by the combination of postural dysfunction and sagittal imbalance. Beside a focus on symptomatic segments, surgical decision making in PD has to address concerns of the sagittal plane. Detailed radiographic analysis in our study stressed that the reconstruction of sagittal balance and its maintenance is imperative for successful surgery in these patients.
References
- 1.Asher SN, Jankovic J. Camptocormia: pathogenesis, classification, and response to therapy. Neurology. 2005;65:355–359. doi: 10.1212/01.wnl.0000171857.09079.9f. [DOI] [PubMed] [Google Scholar]
- 2.Babat LB, McLain RF, Bingaman W, Kalfas L, Young P, Rufo-Smith C. Spinal surgery in patients with Parkinson’s disease: construct failure and progressive deformity. Spine. 2004;29:2006–2012. doi: 10.1097/01.brs.0000138306.02425.21. [DOI] [PubMed] [Google Scholar]
- 3.Barrey C, Jund J, Noseda O, Roussouly P. Sagittal balance of the pelvis–spine complex and lumbar degenerative diseases. A comparative study about 85 cases. Eur Spine J. 2007;16:1459–1467. doi: 10.1007/s00586-006-0294-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benatru I, Vaugoyeau M, Azulay J-P. Postural disorders in Parkinson’s disease. Clin Neurophysiol. 2008;38:459–465. doi: 10.1016/j.neucli.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Berven SH, Deviren V, Smith JA, Emami A, Hu SS, Bradford DS. Management of fixed sagittal plane deformity. Spine. 2001;26:2036–2043. doi: 10.1097/00007632-200109150-00020. [DOI] [PubMed] [Google Scholar]
- 6.Boachie-Adjei O, Dendrinos GK, Ogilvie JW, Transfeldt EE. Management of adult spinal deformity with combined anterior–posterior arthrodesis and Luque–Galveston instrumentation. J Spinal Disord. 1991;4:131–141. doi: 10.1097/00002517-199106000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Boonstra TA, Kooij H, Munneke M, Bloem BR. Gait disorders and balance disturbances in Parkinson’s disease: clinical update and pathophysiology. Curr Opin Neurol. 2008;21:461–471. doi: 10.1097/WCO.0b013e328305bdaf. [DOI] [PubMed] [Google Scholar]
- 8.Boulay C, Tardieu C, Hecquet J, Benaim C, Mouilleseaux B, Marty C, Prat-Pradal D, Legaye J, Duval-Beaupere G, Pelissier J. Sagittal alignment of spine and pelvis: regulated by pelvic incidence: standard values and prediction of lordosis. Eur Spine J. 2006;15:415–422. doi: 10.1007/s00586-005-0984-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daubs MD, lenke LG, Cheh G, Stobbs G, Bridwell KH. Adult spinal deformity surgery. Spine. 2007;32:2238–2244. doi: 10.1097/BRS.0b013e31814cf24a. [DOI] [PubMed] [Google Scholar]
- 10.DeWald CJ, Stanley T. Instrumentation-related complications of multilevel fusions for adult spinal deformity patients over age 65. Surgical considerations and treatment options in patients with poor bone quality. Spine. 2006;31:S144–S151. doi: 10.1097/01.brs.0000236893.65878.39. [DOI] [PubMed] [Google Scholar]
- 11.Dubousset J (1994) Three-dimensional analysis of the scoliotic deformity. In: Weinstein SL (ed) Pediatric spine: principles and practice. Raven Press, New York
- 12.Dupeyron A, Stober N, Gelis A, Castelnovo G, Labauge P, Pélissier (2009) Painful camptocormia: the relevance of shaking your patient’s hand. Eur Spine J. doi:10.1007/s00586-009-1086-6 [DOI] [PMC free article] [PubMed]
- 13.Emami A, Deviren V, Berven S, Smith J, Hu SS, Bradford DS. Outcome and complications of long fusion to the sacrum in adult spinal deformity. Luque–Galveston, combined iliac and sacral screws, and sacral fixation. Spine. 2002;27:776–786. doi: 10.1097/00007632-200204010-00017. [DOI] [PubMed] [Google Scholar]
- 14.Gilad R, Gandhi CD, Arginteanu MS, Moore FM, Steinberger A, Camins M. Uncorrected sagittal plane imbalance predisposes to symptomatic instrumentation failure. Spine J. 2008;8:911–917. doi: 10.1016/j.spinee.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 15.Glassman SD, Berven S, Bridwell KH, Horton W, Dimar JR. Correlation of radiographic parameters and clinical symptoms in adult scoliosis. Spine. 2005;30:682–688. doi: 10.1097/01.brs.0000155425.04536.f7. [DOI] [PubMed] [Google Scholar]
- 16.Glassman SD, Bridwell K, Dimar JR, Horton W, Berven S, Schwab F. The impact of positive sagittal balance in adult spinal deformity. Spine. 2005;30:2024–2029. doi: 10.1097/01.brs.0000179086.30449.96. [DOI] [PubMed] [Google Scholar]
- 17.Grimes J, Hassan M, Trent G, Halle D, Armstrong GW. Clinical and radiographic features of scoliosis in Parkinson’s disease. Adv Neurol. 1987;45:353–355. [PubMed] [Google Scholar]
- 18.Jackson RP, Hales C, Tech COR. Congruent spinopelvic alignment on standing lateral radiographs of adult volunteers. Spine. 2000;25:2808–2815. doi: 10.1097/00007632-200011010-00014. [DOI] [PubMed] [Google Scholar]
- 19.Jackson RP, Kanemura T, Kawakami N, Hales C. Lumbopelvic lordosis and pelvic balance on repeated standing lateral radiographs of adult volunteers and untreated patients with constant low back pain. Spine. 2000;25:575–586. doi: 10.1097/00007632-200003010-00008. [DOI] [PubMed] [Google Scholar]
- 20.Jang JS, Lee SH, Min JH, Maeng DH. Changes in sagittal alignment after restoration of lower lumbar lordosis in patients with degenerative flat back syndrome. J Neurosurg (Spine) 2007;7:387–392. doi: 10.3171/SPI-07/10/387. [DOI] [PubMed] [Google Scholar]
- 21.Johnell O, Sernbo I. Health and social status in patients with hip fractures and controls. Age Ageing. 1986;15:285–291. doi: 10.1093/ageing/15.5.285. [DOI] [PubMed] [Google Scholar]
- 22.Johnell O, Melton LJ, 3rd, Atkinson EJ, O’Fallon WM, Kurland LT. Fracture risk in patients with parkinsonism: a population-based study in Olmsted County, Minnesota. Age Ageing. 1992;21:32–38. doi: 10.1093/ageing/21.1.32. [DOI] [PubMed] [Google Scholar]
- 23.Kao CH, Chen CC, Wang SJ, Chia LG, Yeh SH. Bone mineral density in patients with Parkinson’s disease measured by dual photon absorptiometry. Nucl Med Commun. 1994;15:173–177. doi: 10.1097/00006231-199403000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Karbowski K. The old and the new camptocormia. Spine. 1999;24:1494–1498. doi: 10.1097/00007632-199907150-00017. [DOI] [PubMed] [Google Scholar]
- 25.Kawakami M, Tamaki T, Ando M, Yamada H, Hashizume H, Yoshida M. Lumbar sagittal balance influences the clinical outcome after decompression and posterolateral spinal fusion for degenerative lumbar spondylolisthesis. Spine. 2002;27:59–64. doi: 10.1097/00007632-200201010-00014. [DOI] [PubMed] [Google Scholar]
- 26.Kim YJ, Bridwell KH, Lenke LG, Rhim S, Cheh G. An analysis of sagittal spinal alignment following long adult lumbar instrumented and fusion to L5 or S1: can we predict ideal lumbar lordosis? Spine. 2006;31:2343–2352. doi: 10.1097/01.brs.0000238970.67552.f5. [DOI] [PubMed] [Google Scholar]
- 27.Kim YJ, Bridwell KH, Lenke LG, Rhim S, Cheh G. Pseudoarthrosis in long adult spinal deformity instrumentation and fusion to the sacrum: prevalence and risk factor analysis of 144 cases. Spine. 2006;31:2329–2336. doi: 10.1097/01.brs.0000238968.82799.d9. [DOI] [PubMed] [Google Scholar]
- 28.Koller H, Acosta F, Hempfing A, et al. Long-term investigation of nonsurgical treatment for thoracolumbar and lumbar burst fractures: an outcome analysis in sight of spinopelvic balance. Eur Spine J. 2008;17:1073–1095. doi: 10.1007/s00586-008-0700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Labelle H, Roussouly P, Chopin D, Berthonnaud E, Hresko T, O’Brien M. Spino-pelvic alignment after surgical correction for developmental spondylolisthesis. Eur Spine J. 2008;17:1170–1176. doi: 10.1007/s00586-008-0713-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lafage V, Schwab F, Patel A, Hawkinson N, Farcy J-P. Pelvic tilt and truncal inclination. Two key parameters in the setting of adults with spinal deformity. Spine. 2009;34:E599–E606. doi: 10.1097/BRS.0b013e3181aad219. [DOI] [PubMed] [Google Scholar]
- 31.Lafage V, Schwab F, Skalli W, Hawkinson N, Gagey PM, Ondra S, Farcy JP. Standing balance and sagittal plane spinal deformity: analysis of spinopelvic and gravity line parameters. Spine. 2008;33:1572–1578. doi: 10.1097/BRS.0b013e31817886a2. [DOI] [PubMed] [Google Scholar]
- 32.Laroche M, Delisle M, Aziza R, Lagarrigue J, Mazieres B. Is camptocormia a primary muscular disease? Spine. 1995;20:1011–1016. doi: 10.1097/00007632-199505000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Lazennec JY, Ramaré S, Arafati N, Laudet CG, Gorin M, Roger B, Hansen S, Saillant G, Maurs L, Trabelsi R. Sagittal alignment in lumbosacral fusion: relations between radiological parameters and pain. Eur Spine J. 2002;9:47–55. doi: 10.1007/s005860050008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mok JM, Cloyd JM, Bradford DS, Hu SS, Deviren V, Smith JA, Tay B, Berven SH. Reoperation after primary fusion for adult spinal deformity––rate, reason and timing. Spine. 2009;34:832–839. doi: 10.1097/BRS.0b013e31819f2080. [DOI] [PubMed] [Google Scholar]
- 35.Roussouly P, Gollogly S, Berthonnaud E, Dimnet J. Classification of the normal variation in the sagittal alignment of the human lumbar spine and pelvis in the standing position. Spine. 2005;30:346–353. doi: 10.1097/01.brs.0000152379.54463.65. [DOI] [PubMed] [Google Scholar]
- 36.Schwab F, Dubey A, Gamez L, El Fegoun AB, Hwang K, Pagala M, Farcy JP. Adult scoliosis: prevalence, SF-36, and nutritional parameters in an elderly volunteer population. Spine. 2005;30:1082–1085. doi: 10.1097/01.brs.0000160842.43482.cd. [DOI] [PubMed] [Google Scholar]
- 37.Schwab FJ, Lafage V, Farcy JP, Bridwell KH, Glassman S, Shainline MR. Predicting outcome and complications in the surgical treatment of adult scoliosis. Spine. 2008;33:2444–2447. doi: 10.1097/BRS.0b013e31817d1d4e. [DOI] [PubMed] [Google Scholar]
- 38.Tanguay F, Mac-Thiong J-M, de Guise JA, Labelle H. Relation between the sagittal pelvic and lumbar spine geometries following surgical correction of adolescent idiopathic scoliosis. Eur Spine J. 2006;16:531–536. doi: 10.1007/s00586-006-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tiple D, Fabbrini G, Colosimo C, Ottaviani D, Camerota F, Defazio G, Berardelli A. Camptocormia in Parkinson disease: an epidemiological and clinical study. J Neurol Neurosurg Psychiatry. 2009;80:145–148. doi: 10.1136/jnnp.2008.150011. [DOI] [PubMed] [Google Scholar]
- 40.Tsuchiya K, Bridwell KH, Kuklo TR, Lenke LG, Baldus C. Minimum 5-years analysis of L5–S1 fusion using sacropelvic fixation (bilateral and iliac screws) for spinal deformity. Spine. 2006;31:303–308. doi: 10.1097/01.brs.0000197193.81296.f1. [DOI] [PubMed] [Google Scholar]
- 41.Mummaneni PV, Tumialan LM. Long-segment spinal fixation using pelvic screws. Neurosurgery. 2008;63:S183–S189. doi: 10.1227/01.NEU.0000325680.32776.82. [DOI] [PubMed] [Google Scholar]
- 42.Vialle R, Ilharreborde B, Dauzac C, Lenoir T, Rillardon L, Guigui P. Is there a sagittal imbalance of the spine in isthmic spondylolisthesis? A correlation study. Eur Spine J. 2007;16:1641–1649. doi: 10.1007/s00586-007-0348-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vialle R, Levassor N, Rillardon L, Templier A, Skalli W, Guigui P. Radiographic analysis of the sagittal alignment and balance of the spine in asymptomatic subjects. J Bone Joint Surg. 2005;87-A:260–267. doi: 10.2106/JBJS.D.02043. [DOI] [PubMed] [Google Scholar]
- 44.Weistroffer JK, Perra JH, Lonstein JE, Schwender JD, Garvey TA, Transfeldt EE, Ogilvie JW, Denis F, Winter RB, Wroblewski JM. Complications in long fusions to the sacrum for adult scoliosis: minimum five-year analysis of fifty patients. Spine. 2008;33:1478–1483. doi: 10.1097/BRS.0b013e3181753c53. [DOI] [PubMed] [Google Scholar]