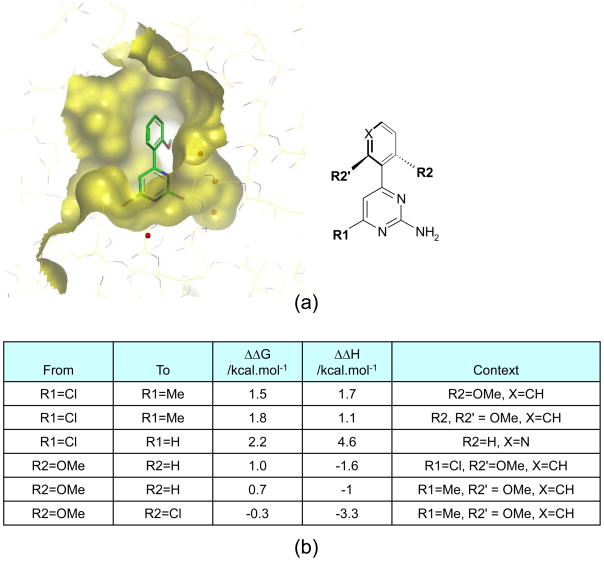
Figure 1. Binding thermodynamics for substituted amino-pyrimidine ligands and HSP90.
(a) X-ray structure showing the binding mode of a substituted amino-pyridimidine bound in the ATP-binding site of the N-terminal domain of the chaperone, HSP90.
(b) Changes in free-energy and enthalpy of binding observed on modification of the amino-pyrimidine ligand. At position R1, replacement of Cl by Me leads to a substantial change in ΔH that is accompanied by a similar change in ΔG. Replacing Cl by H, however leads to a much larger change in ΔH than ΔG indicating a high degree of enthalpy-entropy compensation for this particular substitution. Enthalpy-entropy compensation is also evident for both substitutions shown at position R2. For the substitution of OMe by H, entropy effects dominate and ΔΔG and ΔΔH are of opposite sign. For the substitution of OMe by Cl, enthalpy and entropy changes are finely balanced and little change in ΔG results. With the benefit of enthalpy data is it clear that the substitution OMe to Cl at R2 could lead to large improvements in affinity, provided that enthalpy-entropy compensation could somehow be overcome.