Abstract
Purpose
The aim of this study was to investigate the effect of urinary bladder inflammation on bladder function in a rat chemical cystitis model. We also histologically confirmed the effects of inflammation in the detrusor on chronically inflamed bladder in rats.
Materials and Methods
A total of 13 female Sprague-Dawley rats were used in this study. In seven rats, intravesical instillation of HCl induced chemical cystitis, and the other rats with intravesical instillation of saline were used as the sham. After 2 weeks, cystometrograms were obtained with additional intraabdominal pressure measurements in all unanesthetized, unrestrained rats in metabolic cages. The rats were killed just after cystometry. The bladders were removed and examined histologically for mast cells and inflammatory changes.
Results
The rats with acute injury by HCl showed no differences in pressure parameters, including basal pressure, threshold pressure, and maximum bladder pressure, compared with the sham rats. They showed significantly increased bladder capacity, micturition volume, residual volume, and micturition interval compared with the sham group. They also showed an increased frequency of detrusor overactivity compared with the sham group. The percent of detrusor overactivity was 56.3% among the total intravesical pressure rises above 2 cmH2O. The histological findings of the rats with acute injury by HCl were consistent with chemical cystitis.
Conclusions
Overlapping patterns of lower urinary tract symptoms and pelvic pain are common disease characteristics among interstitial cystitis patients. The situation in an animal model of interstitial cystitis is similar, as observed in this study by the histologic and awake cystometric examinations. However, the interstitial cystitis model showed detrusor overactivity during the filling phase without a decrease in bladder capacity and micturition intervals, which differs from the characteristics of overactive bladder patients.
Keywords: Cystitis, interstitial; Urinary bladder; Cystometry; Rat
Introduction
Interstitial cystitis (IC) is a bladder syndrome characterized by pelvic or lower abdominal discomfort and irritative voiding symptoms such as urinary urgency and frequency and nocturia [1,2]. IC patients frequently have overlapping symptoms related to overactive bladder syndrome, painful bladder syndrome, and chronic pelvic pain syndrome [3]. Clinically, the diagnosis of this condition is based on the presence of pain and bladder irritability. However, IC patients show a wide variation in the severity of both pain and bladder symptoms. Thus, when we investigate the pathophysiology of IC with an animal model for IC, we need to know the simultaneous characteristics of the bladder pathology and the urodynamic findings of IC.
Although a number of potential causal factors have been proposed to explain the pathogenesis of IC, the cause remains uncertain [4]. The possibility remains that there is not a single etiology for the disease, but that the disease is multifactorial. Proposed causative factors include epithelial dysfunction [5], mast cell abnormalities [6], subclinical infection [7], neurogenic inflammation [8], vascular abnormalities [9], and autoimmune phenomena [10]. Animal models for IC have been proposed that take into account the currently available experimental data concerning these factors, but not the clinical bladder symptoms commonly seen in this patient population [11]. Thus, we aimed to investigate the urodynamic findings in an animal model on the basis of the causative factors.
In the present report, we used a well-established model of urinary bladder inflammation using hydrochloric acid (HCl) [12] to examine the consequences of bladder inflammation on urodynamic characteristics in the rat, focusing on their detrusor overactivity during the filling phase.
The purpose of this study was to investigate the effect of urinary bladder inflammation on bladder function during the filling and voiding phases in a rat chemical cystitis model. We also histologically investigated the effects of inflammation in the detrusor on chronically inflamed bladder in rats.
Materials and methods
Animals and study design
A total of 13 female Sprague-Dawley rats (250-300 g; Orient Bio Inc., GyeongGi, Korea) were used in this study. In sevenrats, intravesical instillation of HCl induced chemical cystitis, and the other rats with intravesical instillation of saline were used as the sham. All experimental animal procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and were approved by the local animal ethics committee. The rats were maintained under standard 12:12h light:dark cycles and were allowed access to food pellets and tap water except during experiments. Two weeks after intravesical instillation, cystometrograms were obtained in all unanesthetized, unrestrained rats in metabolic cages. The rats were killed just after cystometry. The bladders were removed and examined histologically for mast cells and inflammatory changes. Rats were anesthetized with ketamine (Ketamine; Yuhan Corp., Seoul, Korea; 75 mg/kg intraperitoneally) and xylazine (Rompun; Bayer Korea Corp, Seoul, Korea; 15 mg/kg intraperitoneally) for specific procedures.
Induction of cystitis
Cystitis was induced by the intravesical instillation of 0.5 ml of 0.4 N HCl, as described by Rivas et al. [13]. Through an abdominal incision made under anesthesia, a 24-gauge needle with a syringe was inserted into the dome of the bladder. After all of the urine was aspirated, HCl was instilled into the bladder lumen for 90 seconds. For the sham group, the same volume of normal saline was instilled.
Procedures for intravesical and intraabdominal catheter implantation
Three days before cystometry, simultaneous catheterizations for intravesical pressure (IVP) and intra-abdominal pressure (IAP) recording were performed as described previously [14]. Briefly, after anesthesia, a polyethylene catheter (PE-50; Becton Dickinson, Parsipanny, NJ, USA) with a cuff was implanted into the dome of the bladder through an abdominal incision. To record IAP, an abdominal balloon (Latex, Dawoo Medical, Incheon, Korea) around the cuff of a catheter tip was placed proximal to the bladder and was tied to the other catheter with silk tie. These catheters were then tunnelled through the subcutaneous space and exited through the back of the animal and anchored to the skin of the back. After surgery, each rat was caged individually and maintained in the same manner.
Functional evaluation
Cystometrograms were obtained in unanesthetized, unrestrained rats in metabolic cages. The indwelling catheter to the bladder was connected to a two-way valve that was connected via a T-tube to a pressure transducer (Research Grade Blood Pressure Transducer; Harvard Apparatus, Holliston, MA, USA) and a microinjection pump (PHD22/2000 pump; Harvard Apparatus). Another indwelling catheter connected to a fluid-filled abdominal balloon was connected to another pressure transducer to record the IAP. Micturition volumes were recorded by means of a fluid collector connected to aforce displacement transducer (Research Grade Isometric Transducer; Harvard Apparatus). Room-temperature saline was infused into the bladder at a rate of 10 ml.h-1. IVP, IAP, and micturition volumes were recorded continuously with Acq Knowledge 3.8.1 software and an MP150 data acquisition system (BIOPAC Systems, Goleta, CA, USA) at a sampling rate of 50 Hz. The mean values from three reproducible micturition cycles were used for evaluation. IAP was defined as the recorded balloon pressure minus the lowest balloon pressure in each voiding cycle (zeroing). Detrusor pressure (DP) was defined as IVP minus IAP. The IVP rises during the filling phase (IVPRs) were defined as increments of IVP that exceeded 2 cm H2O from baseline, which was interpreted as detrusor overactivity (DO) if occurring without simultaneous similar changes in IAP or as AS (abdominal straining)if occurring with simultaneous similar changes in IAP.
Investigation of cystometric parameters
Pressure- and volume-related parameters
Basal pressure (BP; the lowest bladder pressure during filling), threshold pressure (TP; bladder pressure immediately before micturition), maximum pressure (MP; maximum bladder pressure during the micturition cycle), micturition volume (MV; volume of expelled urine), residual volume (RV; remaining urine after voiding), bladder capacity (BC; MV+RV), and micturition interval (MI; intervals between micturition contractions) were measured.
DO-related parameters during the filling phase
The time of filling phase (interval from the initiation of infusion through the tube and the point immediately before the initiation of micturition), frequency of AS per minute, frequency of DO per minute, and increased amplitude from base to peak of the DO spike as IVP were measured. These frequencies were calculated on the basis of the time of filling phase. After cystometry, the animals were sacrificed by cervical dislocation. The bladder and urethra were removed en bloc and separated at the level of the bladder neck and the bladder was weighed.
Histological evaluation
The specimen was split longitudinally, and routine tissue processing for light microscopy was performed. Bladder tissues were embedded in paraffin. Sections (4 µm) were cut by microtome and stained with hematoxylin-eosin (H and E) to assess inflammatory changes with the number of leukocytes and with toluidine blue for mast cells. Slides were examined by an Olympus BX50 light microscope and photographed with an Olympus PM10SP photographic system. Leukocyte infiltration was evaluated to determine the severity of the inflammation that resulted from intravesical instillation.
Statistical analysis
The results are given as mean values±standard errors of the mean (SEM). Normal distributions were confirmed by the Shapiro-Wilks' W test. Statistical significance was determined by paired or unpaired t tests. For multiple comparisons, an ANOVA with Holm-Sidak post-hoc test was used. Statistical significance was considered at p<0.05, p<0.01, and p<0.001. All calculations were made on the basis of n, which denotes the number of animals.
Results
Body weights and bladder weights
There was no significant difference between the sham (245.0±5.2 g) and IC (250.7±3.5 g) groups in body weight 2 weeks after intravesical instillations of saline and HCl. However, urinary bladder weight increased significantly in the IC group (0.13±0.01 g) compared with the sham group (0.11±0.00 g)
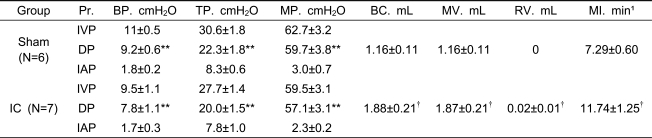
Comparison of pressure-related parameters between the sham and IC groups
There were significant differences between IVP and DP (p<0.01) for all pressure parameters, including BP, TP, and MP, in both groups (Table 1). IC rats showed no significant differences in pressure parameters, including BP, TP, and MP, compared with the sham rats (Table 1, Figure 1). IC rats did not show any significant difference in IAP of BP, TP, and MP compared with the sham rats.
Table 1.
Cystometric pressure and volume parameters in awake, freely moving SD rats in the sham and interstitial cystitis (IC) groups.
Pr.: pressure BP: basal pressure TP: threshold pressure MP: micturition pressure BC: bladder capacity MV: micturition volume RV: residual volume MI: micturition interval IC: interstitial cystitis IVP: intravesical pressure DP: detrusor pressure IAP: intra-abdominal pressure. Results are expressed as mean ±standard error of the mean. *p<0.05, **p<0.01 (unpaired Student's t test), versus IVP; †p<0.05, ††p<0.01 (unpaired Student's t test), versus the sham group.
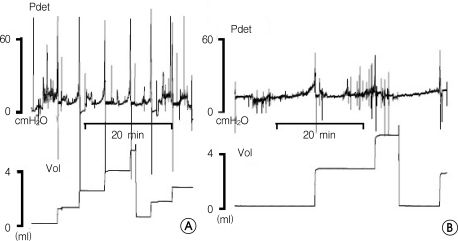
Figure 1.
The rat model of interstitial cystitis (IC) using HCl. A. Sham group at 2 weeks. B. IC model at 2 weeks. The IC model shows an increased micturition interval compared with the sham group.
Comparison of volume-related parameters between the sham and IC groups
The IC group showed significantly increased BC, MV, and MI, except RV, compared with the sham group (Table 1). These findings differ from those of human IC patients.
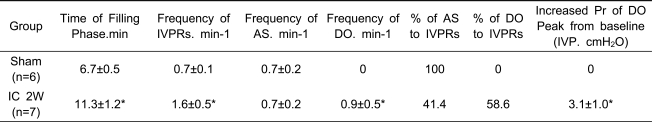
Comparison of DO-related parameters between the sham and IC groups
The IC group showed an increased time of filling phase and total number of IVPRs (sum of the total number of AS and DO) compared with the sham group. IC rats showed a similar frequency of AS, but an increased frequency of DO compared with the sham rats. The percentage of DO was 56.3% among the total IVPRs. The increased intravesical pressures of DO were 3.1±0.1 cmH2O (Table 2).
Table 2.
Characteristics of intravesical pressure rises above 2 cmH2O during the filling phase in awake SD rats in the sham and interstitial cystitis (IC) groups.
IVP: intravesical pressure; IVPR: IVP rises during the filling phase AS: abdominal straining DO: detrusor overactivity Pr.: pressure; IC: interstitial cystitis. Results are expressed as mean ± standard error of the mean. *p<0.05, **p<0.01 (unpaired Student's t test), versus the sham group.
Histologic findings
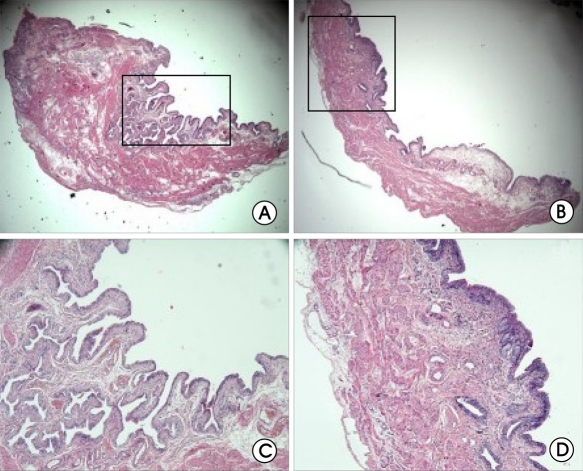
Histologically, the sham-treated bladder specimens showed a normal urothelium, lamina propria, and muscularis (Figure 2). HCl-treated bladder specimens showed a thickened bladder wall and atrophied epithelium, focally consisting of a single cell layer, compared with the shambladder specimens. Histological changes evident after HCl treatment included edema, hemorrhages, and infiltration of inflammatory cells, such as mast cells in the lamina propria, compared with the sham. These findings are consistent with previous reports on chemical cystitis.
Figure 2.
Histologic analysis of rat bladder after HCl irritation. A,E (sham, 2 weeks) B,F (IC, 2 weeks) C,G (sham, 4 weeks) D,H (IC, 4 weeks). Hematoxylin and eosin stain; original magnification ×40 (A-D), ×100 (E-H).
Discussion
IC is a chronic inflammatory disease of the bladder that is characterized by irritative voiding symptoms and pain. Although the etiology and pathogenesis of IC remain obscure, numerous hypotheses, including infection, autoimmune disease, toxic agents from urine, defects of the urothelial lining, and neurogenic causes, have been proposed [5-10]. Several lines of research suggest that inflammation may have an important role in the pathogenesis of this disease, based on the presence of cytokines and chemokines [15], and mast cells may have a key role in the inflammatory response of IC [6]. Defects in mucosal surface glycosaminoglycans and the resulting increased epithelial permeability result in dramatic changes in the micturition reflex circuitry that are characterized by increased excitabilities of reflex connections between the spinal cord and the bladder afferents after bladder inflammation [16]. This is the main mechanism causing the overactive bladder in IC. Another explanation for overactive bladder could be a neuropathic pain originating from the damaged bladder mucosa irritated by a naturally occurring pathologic substance. Irritation of the injured bladder by the hydrostatic pressure of the filled bladder causes this kind of pain and makes the IC patient expel the urine as soon as possible, which is sometimes regarded as neuroplasticity [17,18].
In the animal model in our study, the cascade of events that led to the development of chemical cystitis began with the injury to the bladder epithelium, which wasconfirmed by the histologic findings. According to human studies, the initiating events for the epithelial injury havebeen reported as an episode of bacterial cystitis, and less commonly pelvic surgery, childbirth, or urologic instrumentation. After injury, normal urothelium heals spontaneously, but the uroepithelium from IC patients does not heal appropriately. Furthermore, some reports have suggested that the urine of IC patients contains naturally occurring pathologic substances that inhibit normal urothelial repair, resulting in an inflammation extending to the interstices of the bladder wall. Toxin-induced cystitis has been known to result in dramatic changes in the neurochemical, electrophysiological, and organizational properties of the micturition reflex circuit [4,18-20]. For example, many studies have demonstrated changes in NTF and cytokine mRNA and protein, resulting in changes in the neural activities of the micturition reflex. Some studies have also suggested the involvement of COX-2, prostanoids,and mast cells in cystitis [19,20]. These results all help to explain the underlying mechanisms of the development of overactive bladder in IC patients.
The animal model in our study did not show the usual characteristics of overactive bladder in humans in view of pressure- and volume-related parameters. The IC rats showed no increased BP, TP, or MP or increased BC, MV, RV, or MI compared with the control rats. We suspect that this phenomenon was caused by the basic difference in the structural characteristics of the bladder between humans and rats. Due to the thin and friable characteristics of normal rat bladder, the inflammatory thickening and hardness of the wall may decrease and limit the range of mechanical movement, especially during voiding. After a chronic phase of 2 weeks, the bladder capacity was gradually increased due to the resultant residual urine. However, we could observe the characteristics of overactive bladder in the DO-related parameters. During the filling phase, the total frequency of IVPRs was increased in IC models compared with the controls, among which DO was increased compared with that of control rats. We objectively confirmed, by simultaneous registrations of intravesical and intraabdominal pressure, that DO made up 58.1% of the total IVPR frequency. This is the important observationin the IC model, which shows the overlapping points between pathology and the characteristics of overactive bladder, because the DO corresponds to the urgency in human overactive bladder patients, which is the most important diagnostic clue. When we apply this IC model to future experiments to prove a hypothesis, the present findings will lay important groundwork to prevent misinterpretation of those results.
Conclusions
Overlapping patterns of lower urinary tract symptoms and pelvic pain are common disease characteristics among IC patients. This is similar to the situation in an animal model of IC, as observed in this study by the histologic and awake cystometric examinations. However, the IC model showed detrusor overactivity during the filling phase without a decrease in bladder capacity and micturition intervals, which differs from the characteristics of overactive bladder patients.
Footnotes
This study was supported by a grant of the Korean Health Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea. (A090715)
References
- 1.Moldwin RM. Similarities between interstitial cystitis and male chronic pelvic pain syndrome. Curr Urol Rep. 2002;3:313–318. doi: 10.1007/s11934-002-0056-x. [DOI] [PubMed] [Google Scholar]
- 2.Evans RJ. Treatment approaches for interstitial cystitis: multimodality therapy. Rev Urol. 2002;4(Suppl 1):S16–S20. [PMC free article] [PubMed] [Google Scholar]
- 3.Sant GR. Etiology, pathogenesis, and diagnosis of interstitial cystitis. Rev Urol. 2002;4(Suppl 1):S9–S15. [PMC free article] [PubMed] [Google Scholar]
- 4.Ratliff TL, Klutke CG, McDougall EM. The etiology of interstitial cystitis. Urol Clin North Am. 1994;21:21–30. [PubMed] [Google Scholar]
- 5.Parsons CL, Lilly JD, Stein P. Epithelial dysfunction in nonbacterial cystitis (interstitial cystitis) J Urol. 1991;145:732–735. doi: 10.1016/s0022-5347(17)38437-9. [DOI] [PubMed] [Google Scholar]
- 6.Sant GR, Theoharides TC. The role of the mast cell in interstitial cystitis. Urol Clin North Am. 1994;21:41–53. [PubMed] [Google Scholar]
- 7.Warren JW. Interstitial cystitis as an infectious disease. Urol Clin North Am. 1994;21:31–39. [PubMed] [Google Scholar]
- 8.Pang X, Marchand J, Sant GR, Kream RM, Theoharides TC. Increased number of substance P positive nerve fibres in interstitial cystitis. Br J Urol. 1995;75:744–750. doi: 10.1111/j.1464-410x.1995.tb07384.x. [DOI] [PubMed] [Google Scholar]
- 9.Hohenfellner M, Nunes L, Schmidt RA, Lampel A, Thuroff JW, Tanagho EA. Interstitial cystitis: increased sympathetic innervation and related neuropeptide synthesis. J Urol. 1992;147:587–591. doi: 10.1016/s0022-5347(17)37314-7. [DOI] [PubMed] [Google Scholar]
- 10.Oravisto KJ. Interstitial cystitis as an autoimmune disease. A review. Eur Urol. 1980;6:10–13. doi: 10.1159/000473278. [DOI] [PubMed] [Google Scholar]
- 11.Keay S, Warren JW. A hypothesis for the etiology of interstitial cystitis based upon inhibition of bladder epithelial repair. Med Hypotheses. 1998;51:79–83. doi: 10.1016/s0306-9877(98)90260-2. [DOI] [PubMed] [Google Scholar]
- 12.Cayan S, Coskun B, Bozlu M, Acar D, Akbay E, Ulusoy E. Botulinum toxin type A may improve bladder function in a rat chemical cystitis model. Urol Res. 2003;30:399–404. doi: 10.1007/s00240-002-0291-0. [DOI] [PubMed] [Google Scholar]
- 13.Rivas DA, Chancellor MB, Shupp-Byrne S, Shenot PJ, McHugh K, McCue P. Molecular marker for development of interstitial cystitis in rat model: isoactin gene expression. J Urol. 1997;157:1937–1940. doi: 10.1016/s0022-5347(01)64905-x. [DOI] [PubMed] [Google Scholar]
- 14.Lee T, Andersson KE, Streng T, Hedlund P. Simultaneous registration of intraabdominal and intravesical pressures during cystometry in conscious rats--effects of bladder outlet obstruction and intravesical PGE2. Neurourol Urodyn. 2008;27:88–95. doi: 10.1002/nau.20460. [DOI] [PubMed] [Google Scholar]
- 15.Desireddi NV, Campbell PL, Stern JA, Sobkoviak R, Chuai S, Shahrara S. Monocyte chemoattractant protein-1 and macrophage inflammatory protein-1alpha as possible biomarkers for the chronic pelvic pain syndrome. J Urol. 2008;179:1857–1861. doi: 10.1016/j.juro.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshimura N, de Groat WC. Increased excitability of afferent neurons innervating rat urinary bladder after chronic bladder inflammation. J Neurosci. 1999;19:4644–4653. doi: 10.1523/JNEUROSCI.19-11-04644.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coderre TJ, Katz J, Vaccarino AL, Melzack R. Contribution of central neuroplasticity to pathological pain: review of clinical and experimental evidence. Pain. 1993;52:259–285. doi: 10.1016/0304-3959(93)90161-H. [DOI] [PubMed] [Google Scholar]
- 18.Dupont MC, Spitsbergen JM, Kim KB, Tuttle JB, Steers WD. Histological and neurotrophic changes triggered by varying models of bladder inflammation. J Urol. 2001;166:1111–1118. [PubMed] [Google Scholar]
- 19.Vizzard MA. Increased expression of spinal cord Fos protein induced by bladder stimulation after spinal cord injury. Am J Physiol Regul Integr Comp Physiol. 2000;279:R295–R305. doi: 10.1152/ajpregu.2000.279.1.R295. [DOI] [PubMed] [Google Scholar]
- 20.Vizzard MA, Erdman SL, de Groat WC. Increased expression of neuronal nitric oxide synthase in bladder afferent pathways following chronic bladder irritation. J Comp Neurol. 1996;370:191–202. doi: 10.1002/(SICI)1096-9861(19960624)370:2<191::AID-CNE5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]