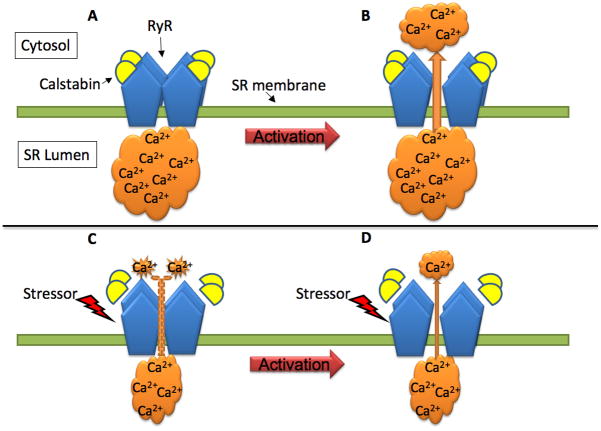
Figure 2. Dissociation of calstabin (FKBP12/FKBP12.6) from the RyR macromolecular complex underlies SR Ca2+ leak. A–B).
Under normal conditions, the interaction between calstabin and the RyR subunits stabilize the channel function and minimizes SR Ca2+ leak when the cell is in resting condition(A). B) Upon activation of the RyR (e.g. by increased [Ca2+]or via interaction with the DHPR), the channel opens and Ca2+ is released from the sarcoplasmic reticulum (SR) into the cytosol, driven by the large SR-cytosolic concentration gradient. C–D) The RyR is a target for stress signals (e.g. β-adrenergic receptor-mediated PKA-dependent phosphorylation of serine residues, nitrosylation of cysteines and oxidative modifications of the RyR). Chronic stress signaling can lead to dissociation of calstabin from the Ca2+ release channel that then becomes leaky, Ca2+ oozes out through the channel and the SR Ca2+ load is diminished (C). Under such conditions, the driving force for Ca2+ across the SR membrane is reduced and upon activation of the RyR less Ca2+ will be released to the cytosol.