Table 1.
Synthesis of desketoraloxifene analogs 3 and 4a
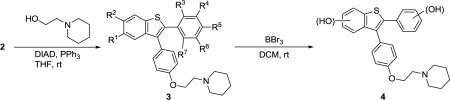
| Entry | 2 | R | Product | 3/4 | Yield (%)d |
|---|---|---|---|---|---|
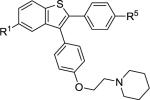
| 1 | 2a | R1,R5 = OMe |
|
3a | 89 |
| 2 | 2a | R1,R5 = OH | 4a b | 58 | |
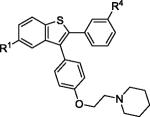
| 3 | 2b | R1,R4 = OMe |
|
3b | 86 |
| 4 | 2b | R1,R4 = OH | 4b b | 61 | |
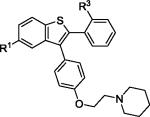
| 5 | 2c | R1,R3 = OMe |
|
3c | 87 |
| 6 | 2c | R1,R3 = OH | 4c b | 52 | |
| 7 | 2d | R1,R4,R6 = OMe |
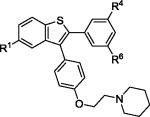
|
3d | 83 |
| 8 | 2d | R1,R4,R6 = OH | 4d c | 39 | |
| 9 | 2e | R2,R5 = OMe |
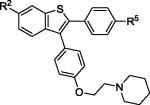
|
3e | 83 |
| 10 | 2e | R2,R5 = OH | 4e (VI) b | 78 | |
| 11 | 2f | R1,R2,R5 = OMe |
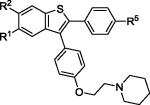
|
3f | 76 |
| 12 | 2f | R1,R2,R5 = OH | 4f c | 41 |
Reagents and conditions: i. Mitsunobu coupling: 2 (0.2 mmol), alkylaminoethanol (1.5 equiv), DIAD (1.5 equiv), PPh3 (2.0 equiv), THF (2.0 mL), rt, 24–36 h. ii. Demethylation: 3 (0.1 mmol), BBr3, CH2C12 (1.0 mL), rt, N2, 3 h.
4.0 Equiv of BBr3 used.
6.0 Equiv of BBr3 used.
Isolated yields after column chromatography. All isolated products were characterized by 1H and 13C NMR spectroscopy (see the Supporting Information).
