Abstract
The transition between adolescence and young adulthood is a developmentally sensitive time where children are at an increased risk for becoming overweight and developing obesity. Twin studies have reported that body mass index [BMI] is highly heritable, however, it remains unclear whether the genetic influences are sex-limited and whether non-additive genetic influences contribute to body mass index [BMI] during these ages. In the current report, we examined self-reported data on BMI in same [n= 2744] and opposite-sex [n = 1178] siblings participating in the National Longitudinal Study on Adolescent Health [Add Health]. To investigate whether the same or different genes contributed to BMI for both sexes, we fit quantitative sex-limited genetic models to three waves of data collection. At each of the three Waves of assessment, models that included additive genetic, individual-specific environment, and no sex-limited genetic influences fit the data most parsimoniously. Heritable effects on BMI at each of the three Waves were large for both sexes and ranged between 0.75 and 0.86. While genetic contributions across the ages were highly correlated, longitudinal analyses indicated that the relevant individual-specific environmental influences on BMI in adolescence and young adulthood change sizably. These results underscore the importance of understanding early genetic influences on BMI and highlight the role environmental experiences have at later ages when new genetic influences appear to make a small contribution to individual variation in BMI.
Introduction
The transition between adolescence and young adulthood is a developmentally sensitive time during which children are at an increased risk for becoming overweight and developing obesity [Anderson and Butcher, 2006]. It is also a critical time for establishing adult weight patterns as many children who are either overweight or obese grow into overweight and obese adults [Crimmins et al, 2007; Gordon-Larson et al, 2004; Freedman et al, 2005; McCarthy et al, 2007]. Similar to adults, overweight and obese children are at a heightened risk for a variety of cardiovascular, pulmonary, and other medical complications, Type II diabetes, and often experience psychological and psychosocial difficulties [Ebbeling et al, 2002; Daniels et al, 2005; Hill et al, 2003; Must et al, 1999]. As such, understanding the etiological influences on weight during these transition years prior to adulthood may inform obesity prevention programs and aid in the development of weight management strategies.
Prevalence rates of adolescents ‘at risk for being overweight’ or overweight has increased over the last decade as has obesity among adults aged 20 years or older [Hedley et al, 2004; Ogden et al, 2006]. For both children and adults, overweight and obesity classifications are typically based on a person’s body mass index [BMI], calculated as weight [kg] / height [m2]. Among children and adolescents aged 2 to 19 years in the United States ‘at risk for being overweight’ and overweight are defined using the age- and sex-specific BMI growth curves from the Centers for Disease Control and Prevention [CDC; Kuczmarski et al, 1990]. BMI between the 5th and 85th percentiles is considered normal, greater-than the 85th but less-than the 95th percentiles ‘at risk for overweight’, and greater-than the 95th percentile overweight or, more recently, obese [Krebs et al, 2007]. By late adolescence [e.g. ages 18 – 20], these percentiles approach those typically used to define adult overweight [BMI >25] and obesity [BMI >30].
An individual’s body mass is a complex, multifactorial trait, influenced by environmental and genetic factors as well as their interaction. Since the early adoption studies by Stunkard and colleagues [Stunkard et al, 1986; Sorensen et al, 1989], familial resemblance for body mass has been shown to be largely due to heritable factors rather than environmental influences shared by siblings [Sorensen et al 1992; Cornes et al, 2007; Maes et al, 1997; Schousboe et al, 2003; Hewitt, 1997; Wardle et al, 2008; Hur et al, 2007]. Estimates of the total genetic contribution to observed variation in BMI have ranged from 0.30 to over 0.90, varying as a function of study design and age [Schousboe et al, 2003; Ordonana et al, 2007; Cornes et al, 2007; Silventoinen et al, 2007; Franz et al, 2007; Pietilainen et al, 1999, 2002; Maes et al, 1997; Wardle et al, 2008; Hur et al, 2007]. Although heritability estimates are medium to large at any single age, the extent to which genetic contributions correlate across ages is less than perfect, indicating possible age-related changes in expressed genetic effects [Franz et al, 2007]. Evidence from time series analyses, though, suggests that there is strong genetic transmission from age-to-age indicating the same genes are contributing to BMI across different ages [Cornes et al, 2007; Silventoinen et al, 2007]. Although seemingly at odds, this dynamic picture of differential genetic expression as a function of age is consistent with the notions of early adiposity rebound [EAR] and critical developmental periods for abnormal weight gain [Rolland-Cachera et al, 2006; Daniels et al, 2005; Taylor et al, 2005; Dietz, 1994].
An additional level of complexity in understanding the impact of hereditary factors on BMI is the role of sex-limited effects. Though present at early ages, there is a marked divergence between males and females in the distribution of fat. As opposed to males, where there are increases in fat-free mass and decreases in body weight due to fat, in females, increases in both are observed [Daniels et al, 2005]. Differences between males and females in weight-related neuroendocrine functioning and fat metabolism have also been described [Bjorntorp, 1997; Hellstrom et al, 2000; Blaak, 2001]. Though only a few studies have been conducted, there is mixed support for sex-limited genetic influences on BMI. In general, positive findings have been reported in adolescent and young adult samples of twins, [Schousboe et al, 2007; Harris et al, 1995; Pietilainen et al, 1999; Cornes et al, 2007], though, not in younger populations [Bodurtha et al, 1990; Hur et al, 2007; Allison et al, 1994]. Sex-limited genetic effects on adult BMI have also been implicated [Schousboe et al, 2007].
In the current report we detail findings from a longitudinal genetic study of BMI. Self-reported height and weight were collected at three time points from adolescent and young adults participating in the National Longitudinal Study of Adolescent Health. Our study was designed to determine: 1) the genetic (additive and non-additive or dominant) and environmental influences on BMI during these transition years, the extent that these etiological factors contributed to variation in BMI, and whether these risk factors differed for males and females, and 2) the extent to which genetic and environmental influences on BMI across different ages were the same or different.
Methods
Subjects
Our sample was drawn from the genetically informative sibling-pairs sample of the larger National Longitudinal study of Adolescent Health [Add Health]. A detailed explanation of the study design and sampling strategy utilized for both the full Add Health and pairs sub-sample is available elsewhere [Harris et al, 2006]. Within the sibling-pairs sample, a total of 5470 individuals participated in the initial in-home interview, with 4984 participating approximately one year later at wave II and 4356 five years later at wave III. Of those reporting, 50.1% to 52.0% were male at each of the three assessments. The mean age at wave I was 16.1, 17.0 at wave II, and 22.4 at wave III. The ethnic composition, based on self-nomination, was 6.9% Asian, 23.7% Black, 1.7% Native American and 67.7% White. Responses from individuals in same-sex pairs were collected from: 544 [M: 284, F: 260] monozygotic [MZ] twins, 511 [M: 279, F: 232] dizygotic [DZ] twins, 1305 [M: 642, F: 663] full-siblings [FS], and 384 [M: 187, F: 197] half-siblings [HS]. Responses from opposite-sex [OS] siblings were also examined and totaled 1178 [OSDZ: 381, OSFS: 454, OSHS: 343]. Zygosity status of the sibling pairs sample was initially determined by self-report at wave I and subsequently at wave III using molecular markers [http://cpc.unc.edu/projects/addhealth/files/biomark.pdf].
Assessment
Self-reported height and weight were collected at each of three Add Health assessments and an index of BMI calculated. For consistency purposes with wave I data we elected not to calculate BMI at waves II and III from measured height and weight. A recent comparison in the larger Add Health data set of self-reported vs. measured weight has shown that self-reports tended to underestimate weight by an average of two pounds [Field et al, 2007] and that self-reported and measured variables correlated 0.92 in the Add Health sample [Goodman et al, 2000]; supporting its validity in community samples. BMI was computed for all subjects who had height and weight data. Exclusions from the current analyses were based on whether: they were currently pregnant, had been pregnant in the prior 12 months [Wave I: n= 148; Wave II: n= 148; Wave III: n= 278], have a physical disability [Wave I: n= 22; Wave II: n= 24; Wave III: n= 50], are an outlier in stature [Wave I: n= 1; Wave II: n= 1; Wave III: n= 4], or there was an excessive decrease in reported height [Wave III: n= 12]. Physical disability was based on whether they were missing a limb, had difficulty with movement or activity, or were reported as being physically disabled by the interviewer. Excessive decrease in reported height was based on height differences of more than 0.2 meters between waves II and III assessments. Some participants met multiple exclusion criteria, for example being both pregnant and physically disabled.
Statistical analyses
Means, variances and sibling correlations for BMI at each assessment Wave were estimated taking into account the non-independence within our data. Age- and sex-differences in sample means and variances were tested using the chi-squared likelihood ratio test with age and sex as covariates and implemented in the freely available software package Mx [Neale et al, 1999].
Quantitative genetic models that estimated the genetic and environmental influences on BMI were conducted using the raw data option with sex and age as covariates. Two genetic models were employed for the current analyses: sex-limitation and Cholesky decomposition [Neale and Cardon, 1992; Neale et al, 2006]. When based on data from same-sex sibling pairs, the sex-limitation model examines whether the magnitude of heritable and environmental contributions to BMI are different between males and females. When data are available from opposite-sex sibling pairs, additional sex-specific genetic and environmental parameters can be included in order to examine whether different risk factors influence BMI in one sex but not the other.
The Cholesky decomposition model was employed to explore the relationship between BMI measurements at different ages. Latent genetic and environmental influences are stratified into those which are common across subsequent measurements and those which are specific or residual to latter ages. For example, latent genetic and environmental factors influencing individual differences in BMI at Wave I are also conceptualized to influences individual differences at Waves II and III. Similarly, latent factors affecting BMI at Wave II would also be conceptualized to influence BMI at Wave III, but not BMI at Wave I.
The fit of our genetic models was evaluated using maximum-likelihood estimation. Our baseline model included the additive genetic [A] and non-shared environmental [E] latent factors and either a non-additive genetic [D] or shared environment [C] factor. The significance of model parameters was evaluated by a comparison of the twice log-likelihood [−2LL] for models with or without the parameters, with the difference distributed as a chi-square distribution and the degrees of freedom being equal to the difference between the number of parameters estimated. A non-significant difference in chi-square [Δχ2] between two models indicates that the parameters dropped from the more parsimonious model were not significantly different from zero. Models were accepted on the basis of the Akaike Information Criterion [AIC] [Akaike, 1987] as calculated by subtracting twice the difference in the degrees of freedom from the difference chi-square between any particular model and the fullest, i.e. least parsimonious, model considered. The AIC indexes the extent that a given model offers the most parsimonious, but adequate, explanation to the data; though limitations to using the AIC as a primary criterion in evaluating model fit do exist (Sullivan and Eaves, 2002).
Results
Based on CDC growth curves, 564 [13.1%; M: 287, F: 277] were above the 85th percentile and 385 [8.9%; M: 219; F: 166] were above the 95th percentile at Wave I and 536 [13.5%; M: 272, F: 264] and 384 [9.7%; M: 216, F: 187] at Wave II, respectively. At Wave III, 913 [26.0%; M: 513, F: 400] participants were overweight and 539 [15.3%; M: 272, F: 267] were obese. Mean BMI scores at Wave I were 22.49 (± 4.38) and 22.15 [± 4.47], for males and females, respectively. At Waves II and III, mean BMI scores were 23.14 [± 4.47] and 25.81 [± 5.22] for males and 22.65 [± 4.83] and 25.36 [± 6.44] for females. Means as a function of twin zygosity are provided in Table 1. The distribution of BMI scores at each of the three Waves of assessment were moderately skewed [skewness > 1.75, kurtosis > 3.0]. As analyses using square-root transformed data did not produce appreciably different results, all analyses presented here are based on raw data. Likelihood ratio tests indicated age and sex effects on BMI and were therefore included as covariates in our analyses.
Table 1.
Means (Standard Deviations) for Body Mass Index during Adolescence and Young Adulthood by Sibling Relatedness.
| Wave 1 (16 years) |
Wave 2 (17 years) |
Wave 3 (22 years) |
||||
|---|---|---|---|---|---|---|
| Males | Females | Males | Females | Males | Females | |
| MZ | 22.23 (4.04) | 21.80 (4.21) | 22.87 (4.08) | 22.05 (4.40) | 25.05 (4.61) | 24.24 (5.79) |
| DZ | 22.23 (3.92) | 21.86 (4.39) | 22.74 (4.15) | 22.44 (4.59) | 25.16 (4.62) | 25.23 (6.06) |
| FS | 22.68 (4.64) | 22.25 (4.62) | 23.46 (4.73) | 22.74 (4.88) | .26.26 (5.50) | 25.59 (6.83) |
| HS | 22.38 (4.38 ) | 22.47 (4.43) | 22.89 (4.27) | 23.35 (5.18) | 25.76 (5.38 ) | 25.75 (6.00) |
| Total | 22.49 (4.38) | 22.15 (4.47) | 23.14 (4.47) | 22.65 (4.83) | 25.81 (5.22) | 25.36 (6.44) |
Abbreviations: MZ, monozygotic twins; DZ, dizygotic twins; FS, full-siblings; HS, half-siblings.
Correlations for same- and opposite-sex siblings are shown in Table 2. In general the MZ twin correlation is roughly twice, and sometimes more than twice, that of the same-sex DZ twin correlation. Though this pattern is consistent with genetic influences on BMI, the larger than expected differences between the MZ/DZ correlations suggests the possibility of non-additive genetic effects. Relative to the MZ twin correlation, the slightly higher combined FS and DZ twin correlation suggests the possibility of shared environmental effects on BMI. Sex-limited effects on BMI are implicated by the lower OSDZ and OSHS correlations than same-sex DZ twin and HS correlations. The greater OSFS than same-sex FS correlations for same-sex female FS at Wave II, however, suggest an absence of sex-limited contributions to BMI.
Table 2.
Correlations (95% Confidence Intervals) for Body Mass Index during Adolescence and Young Adulthood by Sibling Relatedness.
| Wave 1 (16 years) |
Wave 2 (17 years) |
Wave 3 (22 years) |
||||
|---|---|---|---|---|---|---|
| Males | Females | Males | Females | Males | Females | |
| MZ | .85 (.79 – .89) | .85 (.79 – .89) | .85 (.79 – .89) | .71 (.61 – 78) | .84 (.77 – .89) | .83 (.76 – .88) |
| DZ | .31 (.14 – .47) | .45 (.35 – .54) | .27 (.09 – .44) | .44 (.27 – .59) | .32 (.12 – .50) | .23 (.02 – .41) |
| FS | .36 (.15 – .53) | .49 (.39 – .58) | .37 (.26 – .47) | .51 (.30 – .48) | .34 (.21 – .46) | .55 (.43 – .65) |
| HS | .16 (−.07 – .37 ) | .50 (.26 – .68) | .18 (−.05 – .40 ) | .14 (−.12 – .38) | .25 (−.09 – .53 ) | .24 (−.09 – .51) |
| OSDZ | .20 (.05 – .34) | .25 (.08 – .40) | .23 (.02 – .41) | |||
| OSFS | .38 (.29 – .46) | .39 (.30 – .48) | .43 (.32 – .53) | |||
| OSHS | .11 (−.06 – .26) | −.05 (−.22 – .12) | .15 (−.09 – .36) | |||
Abbreviations: MZ, monozygotic twins; DZ, dizygotic twins; FS, full-siblings; HS, half-siblings; OSDZ, opposite sex dizygotic twins; OSFS, opposite sex full-siblings; OSHS, opposite sex half siblings.
Univariate Modeling
Table 3 summarizes the results from our univariate models. Our baseline model allowed A, C, D and E latent factors to be estimated separately for males and females and included sex-specific A factors. The overall fit of this model for BMI at Wave 1 was -2LL = 21866.75 [df = 3856], −2LL = 20222.92 [df = 3491] for Wave II, and −2LL = 17059.47 [df = 2727] for BMI at Wave III. The fit of this baseline model was then compared to the fit of a model that excluded a sex-specific [qualitative sex differences] genetic factor [Model 1]. As shown in Table 3, results indicated that there were not sex-specific genetic influences on BMI during these ages. Models 2 and 3 examined the importance of specifying D and C influences, respectively. Results from these two models indicated that neither non-additive genetic nor shared environmental influences were significant and could be dropped. We next examined whether the magnitudes of genetic and individual-specific environmental influences were different [Model 4] or the same [Model 5] in males and females [quantitative sex differences]. As shown, a model that equated latent A and E factors across the sexes and excluded sex-limited influences offered the most parsimonious fit at Wave I [Model 4]. At Waves II and III, a model that allowed the magnitudes of latent A and E influences to differ between males and females provided the most parsimonious fit [Model 5]. Parameter estimates for the genetic and environmental influences on BMI in males and females from the three Waves are provided in Table 4.
Table 3.
Univariate Fit Statistics for Body Mass Index during Adolescence and Young Adulthood.
| Models | Wave 1 (16 years) |
Wave 2 (17 years) |
Wave 3 (22 years) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Δχ2 | Δdf | p | AIC | Δχ2 | Δdf | p | AIC | Δχ2 | Δdf | p | AIC | ||
| 1. | ADCE, m ≠ f, a′ = 0 | 0.04 | 1 | .84 | −1.96 | 0.61 | 1 | .43 | −1.39 | 0.19 | 1 | .67 | −1.81 |
| 2. | ACE, m ≠ f, a′ = 0 | 0.07 | 3 | .99 | −5.93 | 3.05 | 3 | .38 | −2.95 | 3.44 | 3 | .33 | −2.56 |
| 3. | ADE, m ≠ f, a′ = 0 | 5.10 | 3 | .17 | −0.90 | 2.64 | 3 | .45 | −3.36 | 0.43 | 3 | .93 | −5.57 |
| 4. | AE, m ≠ f, a′= 0 | 5.12 | 5 | .40 | −4.89 | 4.74 | 5 | .45 | −5.26 | 3.44 | 5 | .63 | −6.56 |
| 5. | AE, m = f, a′= 0 | 5.55 | 7 | .59 | −8.45 | 20.48 | 7 | .01 | 6.48 | 25.05 | 7 | .001 | 11.05 |
Note: Δχ2, change in chi square; Δdf, change in degrees-of-freedom; AIC, Akaike Information Criteria; A, additive genetic; C, shared environment; E, Individual-Specific environment, m, male; f, female; a′, sex-specific additive genetic effect.
Bolded: selected as best-fitting model.
Table 4.
Parameter Estimates (95% Confidence Intervals) for the Additive Genetic and Individual Specific Environmental Influences on Body Mass Index during Adolescence and Young Adulthood.
| Wave 1 (16 years) |
Wave 2 (17 years) |
Wave 3 (22 years) |
||||
|---|---|---|---|---|---|---|
| Males | Females | Males | Females | Males | Females | |
| a2 | .84 (.81 – .87) | .86 (.81 – .89) | .75 (.68 – 80) | .85 (.80 – .89) | .83 (.78 – .87) | |
| e2 | .16 (.13 – .19) | .14 (.11 – .19) | .25 (.20 – .32) | .15 (.11 – 20) | .17 (.13 – .22) | |
Note: a2, total genetic influence; e2, total individual-specific environmental influence.
Trivariate Cholesky Decomposition Modeling
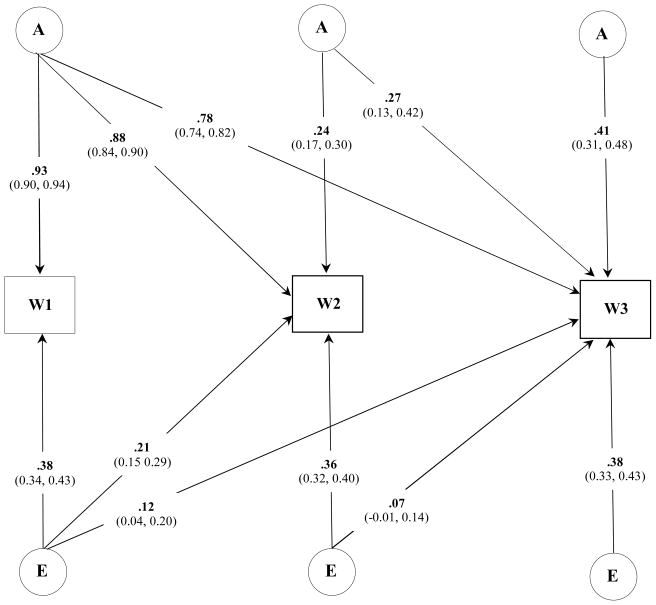
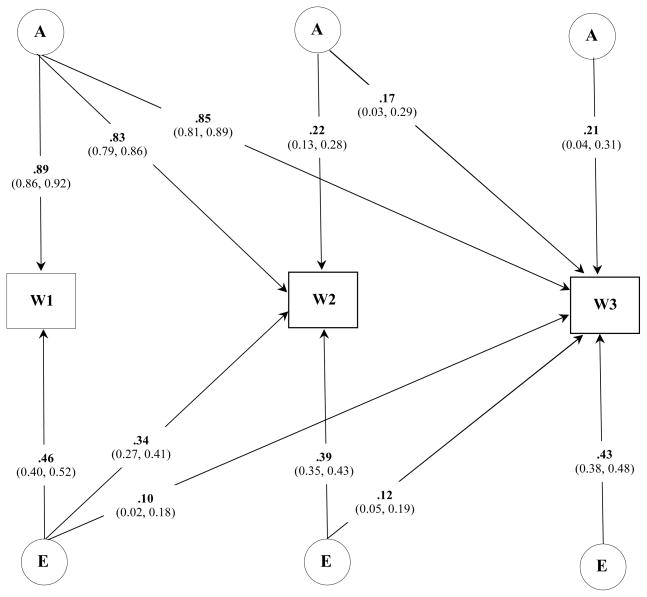
We next investigated the degree of specificity in the genetic and environmental influences on BMI across all three Waves of assessment. The overall fit of a baseline model that specified latent A and E influences that differed between males and females was 54105.97 [df = 10537]. To refine this model, we next examined the fit of a series of nested sub-models that dropped either A or E or both A and E effects within and across each sex. For males, dropping A and E effects individually at Waves II and III resulted in a significant deterioration in model fit (p < .01) and indicated that, though small in overall magnitude, there were new genetic and individual-specific environmental influences on BMI during late adolescence and young adulthood. A similar pattern of results was identified for females. Figures 1 and 2 illustrate the best fitting trivariate model for males and females, respectively.
Figure 1.
Standardized parameter estimates (95% Confidence Intervals) for the best fitting trivariate model of BMI for males across three assessments.
Note: W, wave; A, additive genetic; E, individual specific environmental influences.
Figure 2.
Standardized parameter estimates (95% Confidence Intervals) for the best fitting trivariate model of BMI for females across three assessments.
Note: W, wave; A, additive genetic; E, individual specific environmental influences.
Heritability estimates of BMI in males was 0.85 [CI: 0.81 – 0.89] at Wave I, 0.82 [CI: 0.77 – 0.87] at Wave II, and 0.84 [CI: 0.79 – 0.88] at Wave III. Heritability estimates of BMI in females was 0.79 [CI: 0.73 – 0.84], 0.73 [CI: 0.67 – 0.78], and 0.79 [CI: 0.73 – 0.84], respectively. For both sexes the genetic correlation, or the extent to which genetic influences at one age are the same or different at subsequent ages, were strong (Table 5). Individual specific environmental influences on BMI were more highly correlated across the nine-months between Wave I and II assessments than between BMI at Wave I and III. This suggested that meaningful individual specific environmental effects on BMI change as a person matures into young adulthood.
Table 5.
Genetic and Individual-Specific Environmental Correlations (95% Confidence Intervals) for Body Mass Index.
| Wave1 → Wave 2 | Wave1 → Wave3 | Wave2 → Wave3 | ||
|---|---|---|---|---|
| rg | Males | .96 (.94 – .98) | .85 (.82 – .89) | .89 (.86 – .93) |
| Females | .97 (.95 – .99) | .96 (.92 – .99) | .97 (.93 – 1.0) | |
| re | Males | .51 (.38 – .62) | .30 (.11 – .48) | .29 (.10 – .47) |
| Females | .66 (.56 – .74) | .21 (.05 – .36) | .33 (.20 – .46) |
Note: rg, additive genetic correlation; re, individual specific correlation.
Discussion
Using a large population-based sample of same- and opposite-sex twins and siblings, our goal was to determine the nature and extent of genetic and environmental influences on body mass index [BMI] measured during adolescence and young adulthood. Our results indicate that genetic influences contributed sizably to BMI at all ages but that the magnitudes of their influences were not the same in males and females. Additionally, in both sexes, early expressed genetic effects significantly influenced BMI at later ages. This relationship was strongest for females. Conversely, the influences of individual specific environmental experiences were small to moderate and largely age-specific.
Relationship to Previous Literature
Twin studies of BMI suggest that additive genetic effects contribute to an individual’s risk for becoming overweight and developing obesity [Loos and Bouchard, 2003]. A few studies also suggest that interactions between different alleles within the same gene [e.g. non-additive genetic] may be an important etiological factor [Allison et al, 1994; Neale and Cardon, 1992; Huggins et al, 2000; Cornes et al, 2007]. Both additive genetic and individual-specific environmental experiences are implicated in the stability of BMI across adolescence, young adulthood, and adulthood [Silventoinen et al, 2007; Schousboe et al, 2003; Cornes et al, 2007; Franz et al, 2007]. Heritability estimates have typically been high, ranging between 0.70 and 0.90, with different genetically informative approaches reporting estimates as low as 0.30. Finally, environmental influences shared by siblings from the same family appear to make little contribution to the observed variation in BMI across different ages.
In the current study, univariate models that specified additive genetic [A], individual specific environment [E] effects provided a better and more parsimonious fit than either models that included non-additive genetic [D], shared environmental [C], or sex-limited additive genetic [a′] influences. Consistent with previous findings, heritable influences on BMI at each of the three ages were strong, ranging between 0.84 to 0.86 for males and 0.75 to 0.84 for females. In these data, we were unable to find evidence supporting non-additive genetic contributions. This may, however, be due to the power of our study, as detecting interactions between alleles within the same gene typically requires a larger number of participants than is currently available in the Add Health sibling-pairs sample. A caveat to this interpretation is that in absence of their specification, dominance contributions are subsumed into the estimate of additive genetic effects thus potentially inflating their influence. We also did not find support for shared environmental effects on BMI at any age. Interestingly, one study has reported such contributions to being overweight [>95th percentile] in the Add Health sibling-pairs sample [Nelson et al, 2006]. While potentially due to low statistical power associated with analyzing dichotomous traits, it raises the possibility that shared environmental influences are more pronounced at the extreme ends of the distribution of BMI scores. However, in this same study, a direct comparison of pairs in the same or different households as young adults found no effect of household on BMI and estimated the proportion of variance accounted for by additive genetic effects on BMI ≥ 30 at 0.85.
Differences in the magnitude of heritable contributions between males and females have been reported previously, with males evidencing higher or lower magnitudes than females [Stunkard et al, 1990; Harris et al, 1995; Bodurtha et al, 1990; Allison et al, 1994; Neale and Cardon, 1992; Brook et al, 1975; Hur et al, 2007]. Similar observations have been made in recent studies that have also implicated sex-limited genetic influences [Schousboe et al, 2005; Pietilainen et al, 1999; Cornes et al, 2007]. In these data, we could not equate the genetic and environmental parameters for males and females, though could drop a sex-limited genetic factor from our models. This indicated that a similar set of genetic mechanisms [e.g. quantitative sex differences] are operating in males and females. The observation that the magnitudes of genetic and environmental effects differ between males and females could possibly reflect differences in a number of physiological and biochemical aspects related to BMI and obesity. For example, circulating leptin levels have been shown to be more heritable in males than in females, despite women having higher concentrations at all levels of BMI and a stronger genetic correlation with BMI [Hellstrom et al, 2000; Kaprio et al 2001; Souren et al, 2007]. Finally, males, more than females, evidence higher heritable influences on many features of the metabolic syndrome associated with obesity and its related outcomes [Poulsen et al, 2001].
We investigated the extent of genetic overlap between BMI during adolescence and young adulthood. In these data, expressed heritable influences during adolescence and young adulthood were correlated strongly and indicated that early genetic influences on a persons’ BMI are also impacting BMI at later ages. This interpretation is consistent with other genetic studies reporting correlation coefficients with similar effect sizes [Silventoinen et al, 2007; Cornes et al, 2007] as well as a potential explanation of the stability of BMI observed in epidemiological studies [Gordon-Larson et al, 2004]. Additionally, our trivariate analyses suggest that the relevant environmental influences on BMI in adolescence and young adulthood change sizably. Changing dietary behaviors as well as food sources [Powell et al, 2007; Binkley et al, 2000] during adolescence may be potentially relevant individual-specific environmental factors. Individual choices and attitudes towards diet and health may also be relevant as their influences on BMI appear to be different between the sexes [Kuchler and Lin, 2002] and may be an important factor in prevention and intervention efforts directed towards females during mid- to-late adolescence. However, the increased individual-specific environmental influences for females during these ages may also point to role of peer influence on maintaining a certain weight.
Limitations
While our results are consistent with a number of previous findings, they should be interpreted in light of a number of limitations. First, while BMI is higher in the United States as compared with the rest of the world, rates of BMI within the US can vary substantially [Mokdad et al, 1999]. As participants in the sibling-pairs sample are drawn from around the country, regional variation may be a factor in our results. Equally, BMI rates are higher in rural areas than in urban areas of the US [Jackson et al, 2005] and together with regional variation may account for our slightly lower estimate of obesity (13.1%) than reported in the NHANES study (15.5%; Odgen et al, 2002). To our knowledge there has not been a genetically informative study of BMI along these lines. Second, the sibling-pairs sample is ethnically heterogeneous; with Caucasians accounting for approximately 70% of the sample. In our analyses we did not include ethnicity as a covariate as it was determined in regression analyses not to be a predictor of BMI after controlling for the influence of age and sex. Therefore, our results may not generalize to samples with different ethnic compositions. Third, we treated BMI as a continuous or quantitative measure and did not take into account the clinically relevant differences within the range of BMI’s observed in this sample. Though this is a more statistically powerful approach and is consistent with previous studies [Schousboe et al, 2003; Ordonana et al, 2007; Cornes et al, 2007; Silventoinen et al, 2007; Franz et al, 2007; Pietilainen et al, 1999, 2002; Maes et al, 1997] there has been a suggestion that shared environmental influences are relevant to the clinical classifications of overweight and obese [Nelson et al, 2006]. Lastly, though sample sizes were large in the current study, our results did not support previous reports of non-additive genetic contributions to BMI. The potential role for interacting loci in the etiology of BMI is supported, however, by results from genome-wide linkage scans which implicate multiple chromosomal regions [Cornes et al, 2005; Sanders et al, 2007; Deng et al, 2002; Snyder et al, 2004; Dong et al, 2005] and candidate gene association analyses of the beta-adrenergic receptors [Ellsworth et al, 2005]. As such, future studies with larger samples sizes and thus greater statistical power to detect non-additive genetic effects, should parameterize such influences in their models of BMI.
Acknowledgments
This research uses data from Add Health, a program project designed by J. Richard Udry, Peter S. Bearman, and Kathleen Mullan Harris, and funded by a grant P01-HD31921 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, with cooperative funding from 17 other agencies. Special acknowledgement is due Ronald R. Rindfuss and Barbara Entwisle for assistance in the original design. Persons interested in obtaining data files from Add Health should contract Add Health, Carolina Population Center, 123 W. Franklin Street, Chapel Hill, NC, 275-2524 (addhealth@unc.edu). BCH, JKH, JML, and CJH were supported by grant HD031921. CJH was also supported by grant R01-DA021913, MBM was supported by grant DA011015, AS was supported by grant HD319121, JDB was supported by grant K01-HD50336.
References
- Akaike H. Factor analyses and AIC. Psychometrika. 1987;52:317–322. [Google Scholar]
- Allison DB, Heshka S, Neale MC, Lykken DT, Heymsfield SB. A genetic analysis of relative weight among 4,020 twin pairs, with an emphasis on sex effects. Health Psychol. 1994;13:362–365. doi: 10.1037//0278-6133.13.4.362. [DOI] [PubMed] [Google Scholar]
- Anderson PM, Butcher KF. Childhood obesity: Trends and potential causes. The Future of Children: Childhood Obesity. 2006;16(1):19–46. doi: 10.1353/foc.2006.0001. [ www.futureofchildren.org] [DOI] [PubMed]
- Bjorntorp P. Hormonal control of regional fat distribution. Hum Reprod. 1997;1:S21–S25. doi: 10.1093/humrep/12.suppl_1.21. [DOI] [PubMed] [Google Scholar]
- Blaak E. Gender differences in fat metabolism. Curr Opin Clin Nutr Metab Care. 2001;4:499–502. doi: 10.1097/00075197-200111000-00006. [DOI] [PubMed] [Google Scholar]
- Blinkley JK, Eales J, Jekanowski M. The relation between dietary change and rising US obesity. Int J Obesity. 2000;24:1032–1039. doi: 10.1038/sj.ijo.0801356. [DOI] [PubMed] [Google Scholar]
- Bodurtha JN, Mosteller M, Hewitt JK, Nance WE, Eaves LJ. Genetic analysis of anthropometric measures in 11-year old twins: The Medical College of Virginia Twin Study. Pediatr Res. 1990;28:1–4. doi: 10.1203/00006450-199007000-00001. [DOI] [PubMed] [Google Scholar]
- Brook GD, Huntley RMC, Slack J. Influence of heredity and environment in the determination of skinfold thickness in children. BMJ. 1975;2:719–721. doi: 10.1136/bmj.2.5973.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornes BK, Zhu G, Martin NG. Sex differences in genetic variation in weight: a longitudinal study of body mass index in adolescent twins. Behav Genet. 2007;37:648–660. doi: 10.1007/s10519-007-9165-0. [DOI] [PubMed] [Google Scholar]
- Crimmins NA, Dolan LM, Martin LJ, Bean JA, Daniels SR, Lawson ML, Goodman E, Woo JG. Stability of adolescent body mass index during three years of follow-up. J Pediatrics. 2007;151:373–387. doi: 10.1016/j.jpeds.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Daniels SR, Arnett DK, Eckel RH, Gidding SS, Hayman LL, Kumanyika S, Robinson TN, Scott BJ, St Jeor S, Williams CL. Overweight in children and adolescents: Pathophysiology, consequences, prevention and treatment. Circulation. 2005;111:1999–2012. doi: 10.1161/01.CIR.0000161369.71722.10. [DOI] [PubMed] [Google Scholar]
- Deng HW, Deng H, Liu YJ, Liu YZ, Xu FH, Shen H, Conway T, Li JL, Huang QY, Davies KM, Recker RR. A genomewide linkage scan for quantitative-trait loci for obesity phenotypes. Am J Hum Genet. 2002;70:1138–1151. doi: 10.1086/339934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz WH. Critical periods in childhood for the development of obesity. Am J Clin Nutr. 1994;59:955–959. doi: 10.1093/ajcn/59.5.955. [DOI] [PubMed] [Google Scholar]
- Dong C, Li WD, Li D, Price A. Interaction between obesity-susceptibility loci in chromosome regions 2p25-p24 and 13q13-q21. Eur J Hum Genet. 2005;13:102–108. doi: 10.1038/sj.ejhg.5201292. [DOI] [PubMed] [Google Scholar]
- Ebbelin CB, Pawlak DB, Ludwig DS. Childhood obesity: public-health crisis, common sense cure. Lancet. 2002;360:473–482. doi: 10.1016/S0140-6736(02)09678-2. [DOI] [PubMed] [Google Scholar]
- Ellsworth DL, Coady SA, Chen W, Srinivasan SR, Boerwinkle E, Berenson GS. Interactive effects between polymorphisms in the β-adernergic receptors and longitudinal changes in obesity. Obesity Res. 2005;13:519–526. doi: 10.1038/oby.2005.55. [DOI] [PubMed] [Google Scholar]
- Field AE, Aneja P, Rosner B. The validity of self-reported weight change among adolescents and young adults. Obesity. 2007;15:2357–2364. doi: 10.1038/oby.2007.279. [DOI] [PubMed] [Google Scholar]
- Franz CE, Grand MD, Jacobson KC, Kremen WS, Eisen SA, Xian H, Romeis J, Thompson-Brenner H, Lyons MJ. Genetics of body mass index stability and risk for chronic disease: A 28-year longitudinal study. Twin Res Hum Genet. 2007;10:537–545. doi: 10.1375/twin.10.4.537. [DOI] [PubMed] [Google Scholar]
- Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, Berenson GS. The relation of childhood BMI to adult adiposity: The Bogalusa Heart Study. Pediatrics. 2005;115:22–27. doi: 10.1542/peds.2004-0220. [DOI] [PubMed] [Google Scholar]
- Goodman E, Hinden BR, Khandelwal S. Accuracy of teen and parental reports of obesity and body mass index. Pediatrics. 2000;106:52–58. doi: 10.1542/peds.106.1.52. [DOI] [PubMed] [Google Scholar]
- Gordon-Larsen P, Adair LS, Nelson MC, Popkin BM. Five-year obesity incidence in the transition period between adolescence and adulthood: the National Longitudinal Study of Adolescent Health. Am J Clin Nutr. 2004;80:569–575. doi: 10.1093/ajcn/80.3.569. [DOI] [PubMed] [Google Scholar]
- Harris KH, Halperrn CT, Smolen A, Haberstick BC. The National Longitudinal Study of Adolescent Health (Add Health) twin data. Twin Res Hum Genet. 2006;9:988–997. doi: 10.1375/183242706779462787. [DOI] [PubMed] [Google Scholar]
- Harris JR, Tambs K, Magnus P. Sex-specific effects for body mass index in the new Norwegian twin panel. Genet Epidemiol. 1995;12:251–265. doi: 10.1002/gepi.1370120303. [DOI] [PubMed] [Google Scholar]
- Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents and adults, 1999–2002. JAMA. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- Hellstrom L, Wahrenberg H, Hruska K, Reynisdottir S, Arner P. Mechanisms behind gender differences in circulating leptin levels. J Intern Med. 2000;247:457–462. doi: 10.1046/j.1365-2796.2000.00678.x. [DOI] [PubMed] [Google Scholar]
- Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science. 2003;299:853–855. doi: 10.1126/science.1079857. [DOI] [PubMed] [Google Scholar]
- Huggins RM, Hoang NH, Loesch DZ. Analysis of longitudinal data from twins. Genet Epidemiol. 2000;19:345–353. doi: 10.1002/1098-2272(200012)19:4<345::AID-GEPI6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Hur YM. Sex differences in heritability of BMI in South Korean adolescent twins. Obesity. 2007;15:2908–2911. doi: 10.1038/oby.2007.346. [DOI] [PubMed] [Google Scholar]
- Jackson JE, Doescher MP, Jerant AF, Hart LG. A national study of obesity prevalence and trends by type of rural county. J Rural Health. 2005;21:140–148. doi: 10.1111/j.1748-0361.2005.tb00074.x. [DOI] [PubMed] [Google Scholar]
- Kaprio J, Eriksson J, Lehtovitra M, Koskenvuo M, Tuomilehto J. Heritability of leptin levels and the shared genetic effects on body mass index and leptin in adult Finnish twins. Int J Obesity. 2001;25:132–137. doi: 10.1038/sj.ijo.0801526. [DOI] [PubMed] [Google Scholar]
- Krebs NF, Himes JH, Jacobson D, Nicklas TA, Guilday P, Styne D. Assessment of child and adolescent overweight and obesity. Pediatrics. 2007;120:S193–S228. doi: 10.1542/peds.2007-2329D. [DOI] [PubMed] [Google Scholar]
- Kuchler F, Lin BH. The influence of individual choices and attitudes on adiposity. Int J Obesity. 2002;26:1017–1022. doi: 10.1038/sj.ijo.0802009. [DOI] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Grummer-Strawn M, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. CDC Growth Charts: United States. Centers for Disease Control and Prevention, National Center for Health Statistics; 2000. [accessed 23 October, 2007]. Internet: http://www.cdc.gov/growthcharts. [Google Scholar]
- Loos RJF, Bouchard C. Obesity – is it a genetic disorder? J Intern Med. 2003;254:401–425. doi: 10.1046/j.1365-2796.2003.01242.x. [DOI] [PubMed] [Google Scholar]
- Maes HH, Neale MC, Eaves LJ. Genetic and environmental factors in relative body weight and human adiposity. Behav Genet. 1997;27:325–352. doi: 10.1023/a:1025635913927. [DOI] [PubMed] [Google Scholar]
- McCarthy A, Hughes R, Tilling K, Davies D, Smith GD, Ben-Shlomo Y. Birth weight: postnatal, infant, and childhood growth; and obesity in young adulthood: evidence from the Barry Caerphilly Growth Study. Am J Clin Nutr. 2007;86:907–913. doi: 10.1093/ajcn/86.4.907. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Serdula MK, Dietz WH, Bowman BA, Marks JS, Koplan JP. The spread of the obesity epidemic in the United States, 1991–1998. JAMA. 1999;282:1519–1522. doi: 10.1001/jama.282.16.1519. [DOI] [PubMed] [Google Scholar]
- Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- Neale MC, Cardon LR. Methodology for Genetic Study of Twins and Families. Dordrecht: Kluwer Academic Publishers; 1992. [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes H. Mx: Statistical Modeling. Box 126 MCV, Richmond, VA 23298: Department of Psychiatry; 1999. [Google Scholar]
- Neale MC, Roysamb E, Jacobson K. Multivariate genetic analysis of sex limitation and GxE interaction. Twin Res Human Genet. 2006;9:481–489. doi: 10.1375/183242706778024937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MC, Gordon-Larsen P, North KE, Adair LS. Body mass index gain, fast food and physical activity: effects of shared environment over time. Obesity. 2006;14:701–709. doi: 10.1038/oby.2006.80. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- Ordonana JR, Rebollo-Mesa I, Gonzalez-Javier R, Perez-Riquelme F, Martinez-Selva JM, Willemsen G, Boomsma DI. Heritability of body mass index: A comparison between the Netherlands and Spain. Twin Res Hum Genet. 2007;10:749–756. doi: 10.1375/twin.10.5.749. [DOI] [PubMed] [Google Scholar]
- Pietlainen KH, Kaprio J, Rissanen A, Winter T, Rimpela A, Viken RJ, Rose RJ. Distribution and heritability of BMI in Finnish adolescents aged 16y and 17y: A study of 4884 twins and 2509 singletons. Int J Obesity. 1999;23:107–115. doi: 10.1038/sj.ijo.0800767. [DOI] [PubMed] [Google Scholar]
- Pietlainen KH, Kaprio J, Rasanen M, Rissanen A, Rose RJ. Genetic and environmental influences on the tracking of body size from birth to early adulthood. Obesity Res. 2002;10:875–884. doi: 10.1038/oby.2002.120. [DOI] [PubMed] [Google Scholar]
- Poulsen P, Vaag A, Kyvik K, Beck-Nielsen H. Genetic versus environmental aetiology of the metabolic syndrome among male and female twins. Diabetologia. 2001;44:537–543. doi: 10.1007/s001250051659. [DOI] [PubMed] [Google Scholar]
- Powell LM, Auld C, Chaloupka FJ, O’Malley PM, Johnston LD. Associations between access to food stores and adolescent body mass index. Am J Prev Med. 2007;33:S301–S307. doi: 10.1016/j.amepre.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Rolland-Cachera MF, Deheeger M, Maillot M, Bellisle F. Early adiposity rebound: causes and consequences for obesity in children and adults. Int J Obesity. 2006;30:S11–S17. doi: 10.1038/sj.ijo.0803514. [DOI] [PubMed] [Google Scholar]
- Saunders CL, Chiodini BD, Sham P, Lewis CM, Abkevich V, Adeyemo AA, de Andrade M, Arya R, Berenson GS, Blangero J, Boehnke M, Borecki IB, Chagnon YC, Chen W, Comuzzie AF, Deng HW, Duggirala R, Feitosa MF, Froguel P, Hanson RL, Hebebrand J, Huezo-Dias P, Kissebah AH, Li W, Luke A, Martin LJ, Nash M, Ohman M, Palmer LJ, Peltonen L, Perola M, Price RA, Redline S, Srinivasan SR, Stern MP, Stone S, Stringham H, Turner S, Wijmenga C, Collier DA. Meta-analysis of genome-wide linkage studies in BMI and obesity. Obesity. 2007;15:2263–2275. doi: 10.1038/oby.2007.269. [DOI] [PubMed] [Google Scholar]
- Schousboe K, Willemsen G, Kyvik KO, Mortensen J, Boomsma DI, Cornes BK, Davis CJ, Fagnani C, Hjelmborg J, Kaprio J, de Lange M, Luciano M, Martin NG, Pedersen N, Pietlainen KH, Rissanen A, Saarni S, Sorensen TIA, van Ball GCM, Harris JM. Sex differences in heritability of BMI: A comparative study of results from twin studies in eight countries. Twin Res Hum Genet. 2003;6:409–421. doi: 10.1375/136905203770326411. [DOI] [PubMed] [Google Scholar]
- Silventoinen K, Pietlainen KH, Tynelius P, Sorensen TIA, Kaprio J, Rasmussen F. Genetic and environmental factors in relative weight from birth to age 18: The Swedish Young Male Twins Study. Int J Obesity. 2007;31:615–621. doi: 10.1038/sj.ijo.0803577. [DOI] [PubMed] [Google Scholar]
- Sorensen TIA, Holst C, Stunkard AJ. Childhood body mass index – genetic and familial environmental influences assessed in a longitudinal adoption study. Int J Obes Relat Metab Disord. 1992;16:705–714. [PubMed] [Google Scholar]
- Sorensen TIA, Price RA, Stunkard AJ, Schulsinger F. Genetics of obesity in adult adoptees and their biological siblings. Br Med J. 1989;298:87–89. doi: 10.1136/bmj.298.6666.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Eaves LJ. Evaluation of the analysis of univariate discrete twin data. Behav Genet. 2002;32:221–227. doi: 10.1023/a:1016025229858. [DOI] [PubMed] [Google Scholar]
- Souren NY, Paulussen ADC, Loos RJF, Gielen M, Beunen G, Fagard R, Derom C, Vlietinck R, Zeegers MP. Anthropometry, carbohydrate and lipid metabolism in the East Flanders Prospective Twin Survey: heritabilities. Diabetologia. 2007;50:2107–2116. doi: 10.1007/s00125-007-0784-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stunkard AJ, Sorensen TI, Hanis C, Teasdale TW, Chakraborty R, Schull WJ, Schulsinger F. An adoption study of human obesity. N Engl J Med. 1986;314:193–198. doi: 10.1056/NEJM198601233140401. [DOI] [PubMed] [Google Scholar]
- Stunkard AJ, Harris JR, Pedersen NL, McClearn GE. The body-mass index of twins who have been reared apart. 1990;322:1483–1487. doi: 10.1056/NEJM199005243222102. [DOI] [PubMed] [Google Scholar]
- Taylor RW, Grant AM, Goulding A, Williams SM. Early adiposity rebound: review of papers linking this to subsequent obesity in children and adults. Curr Opin Clin Nutr Metab Care. 2005;8:607–612. doi: 10.1097/01.mco.0000168391.60884.93. [DOI] [PubMed] [Google Scholar]
- Wardle J, Carnell S, Haworth CMA, Plomin R. Evidence for a strong genetic influence on childhood adiposity despite the force of the obesogenic environment. Am J Clin Nutr. 2008;87:398–404. doi: 10.1093/ajcn/87.2.398. [DOI] [PubMed] [Google Scholar]
- Zeegers MP. Anthropometry, carbohydrate and lipid metabolish in the East Flanders Prospective Twin Survey: heritabilities. Diabetologia Epub. 2007 doi: 10.1007/s00125-007-0784-z. [DOI] [PMC free article] [PubMed] [Google Scholar]