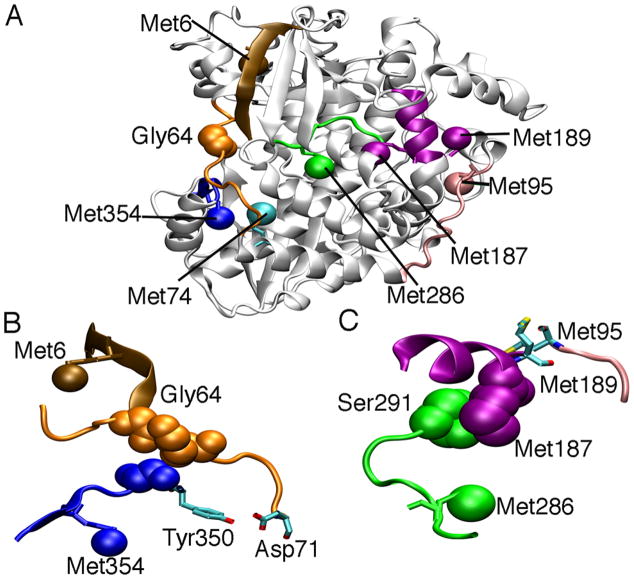
Figure 6.
Transmission of long distance signals across the protein structure of PV 3Dpol. A. Mutation of Gly64 to Ser affects the structure and/or dynamics of distant amino acids, including Met95, Met187 and Met189 on the far side of the protein. B. The α1-α2 loop (orange) interacts with motif D (blue), including a hydrogen bond interaction between the sidechains of Asp71 and Tyr350. C. Met187 interacts with residues on the β10-α13 loop of motif B, including Ser291. This suggests that Met187 is an indirect probe for the structure/dynamics of the β10-α13 loop. The backbone amide of Met95 hydrogen bonds to the backbone carbonyl of Met189. These interactions, including the hydrogen bonding network outlined in Figure 1B, allows the transmission of signals from Gly64 all the way to Met95 over 30 Å away.