Figure 4.
Characterization of Plant AAH Proteins.
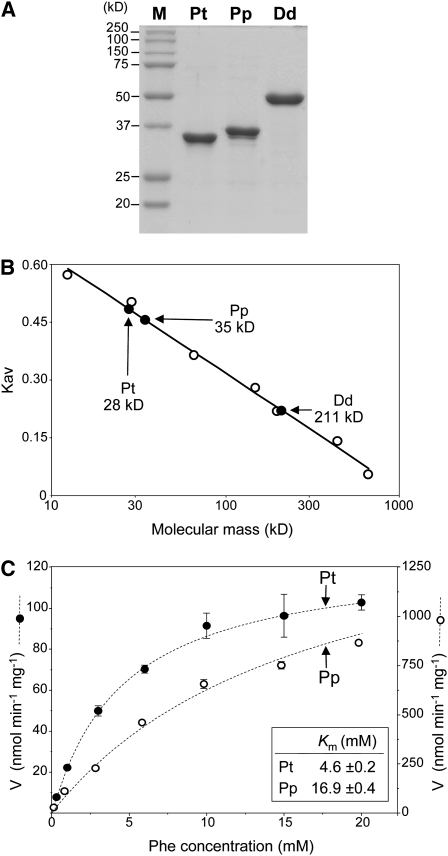
(A) Purification of recombinant P. taeda (Pt) and P. patens (Pp) AAHs and D. discoideum (Dd) Phe hydroxylase. Gel lanes contained 5 μg of Ni affinity-purified protein. Proteins were separated by denaturing gel electrophoresis and stained with Coomassie blue. M, molecular mass markers.
(B) Analytical size exclusion chromatography of P. taeda AAH, P. patens AAH, and D. discoideum Phe hydroxylase. The calculated molecular masses of the monomers are 35, 37, and 51 kD, respectively. Open circles are calibration standards (kD): cytochrome c (12.4), carbonic anhydrase (29), albumin (66), alcohol dehydrogenase (150), β-amylase (200), apoferritin (443), and thyroglobulin (669).
(C) Velocity versus Phe concentration curves for P. patens and P. taeda AAHs; the H4BPt cofactor concentration was 500 μM. Data are means and se of three replicates. Error bars smaller than the points are omitted.