Figure 2.
Subcellular Localization of PUB1 and Interaction with LYK3 in Planta.
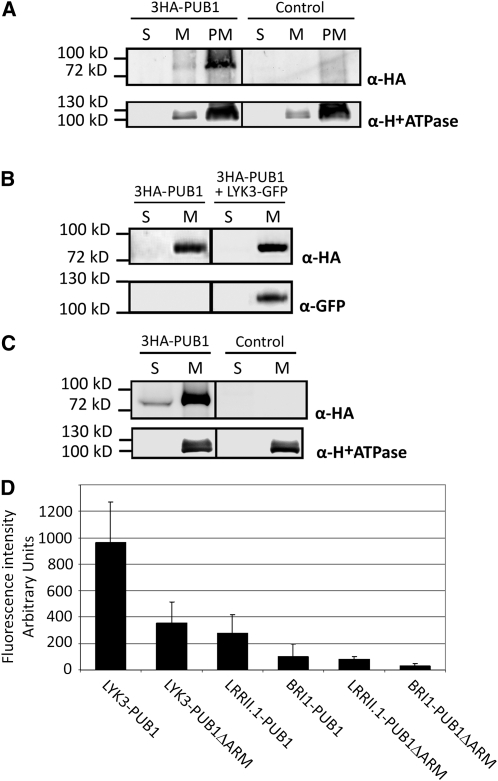
For all biochemical experiments ([A] to [C]), plant material was fractionated into plasma membrane (PM), total membrane (M), or soluble (S) fractions. S and M fractions were concentrated and resuspended in the same volume and equal volumes were loaded. M and PM fractions contained equal protein amounts. The three fractions were used in immunoblots with indicated antibodies.
(A) 3HA-PUB1 is associated with the plasma membrane in N. benthamiana. N. benthamiana leaves were transformed with the 3HA-PUB1 construct or the vector alone (control).
(B) The membrane localization of PUB1 is not altered by coexpression with LYK3 in N. benthamiana. N. benthamiana leaves expressed 3HA-PUB1 alone or together with LYK3-GFP.
(C) 3HA-PUB1 is predominantly associated with membranes in M. truncatula and is also present in the soluble fraction. M. truncatula roots were transformed with either the 3HA-PUB1 construct or control vector expressing GFP.
(D) PUB1 interacts with LYK3 and not with BRI1 or LRRII.1 in N. benthamiana. BiFC experiments were performed by coexpression of the indicated split-YFP pair combinations: The C-terminal domain of YFP (Yc) was fused to the C terminus of the RLKs, whereas the N-terminal domain of YFP (Yn) was fused to the N terminus of PUB1 or a derivative lacking the ARM repeats (PUB1ΔARM). Leaves were imaged by epifluorescence using identical exposure settings and quantified. Fluorescence intensities (arbitrary units) and standard deviations are shown.