Figure 3.
In Vitro E3 Ubiquitin Ligase Activity of PUB1, Trans-Phosphorylation of PUB1 by LYK3, and Coexpression Studies of PUB1 and LYK3 in N. benthamiana.
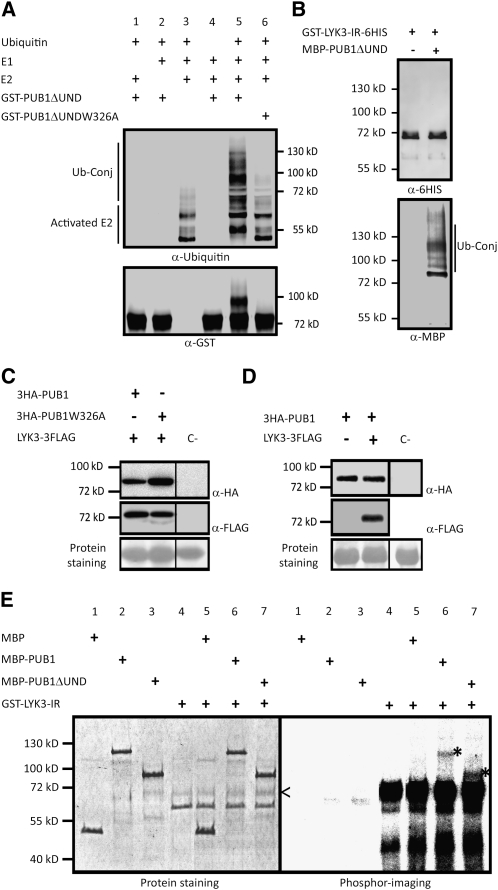
(A) PUB1 possesses an E3 ubiquitin ligase activity in vitro. GST-PUB1ΔUND was tested for E3 ubiquitin ligase activity in presence or absence of yeast E1, Arabidopsis E2 (UBC8), and ubiquitin. Anti-Ub and anti-GST antibodies were used to detect ubiquitinated proteins and GST-PUB1ΔUND, respectively. A range of proteins conjugated with ubiquitin (Ub-conj) were formed when GST-PUB1ΔUND was added in the mix and not with GST-PUB1ΔUND-W326A, an inactive U-box variant.
(B) LYK3 in vitro ubiquitination assay. GST-LYK3-IR-6HIS with E1, E2, and ubiquitin was incubated in the presence or absence of MBP-PUB1ΔUND. Immunoblot analyses were performed with indicated antibodies.
(C) PUB1 does not affect LYK3 abundance in N. benthamiana. 3HA-PUB1 or the inactive 3HA-PUB1W326A mutated protein was coexpressed with LYK3-3FLAG. Protein combinations were expressed in different halves of the same N. benthamiana leaf to avoid leaf expression effects. Total extracts from 1-cm diameter leaf discs were analyzed by immunobloting with the corresponding antibodies. Nontransformed leaves were used as the immunoblotting control (C-). Ponceau red staining shows that equal amount of protein were loaded on the gel.
(D) LYK3 does not affect PUB1 abundance in N. benthamiana. 3HA-PUB1 was expressed with or without LYK3-3FLAG. Experiments were performed as in (C).
(E) PUB1 is phosphorylated by LYK3 in vitro. The auto- and trans-phosphorylation activities of GST-LYK3 (intracellular region) were assayed alone or by addition of MBP, MBP-PUB1, or MBP-PUB1ΔUND. On the left panel, protein staining shows the size and quantity of the purified proteins. On the right panel, phosphor imaging reveals LYK3 kinase-dependent phosphorylation. The ~70-kD recombinant LYK3 kinase (<) as detected by immunoblot using anti-GST antibodies (see Supplemental Figure 3 online) shows autophosphorylation. Trans-phosphorylated forms of MBP-PUB1 and MBP-PUB1ΔUND are visible at the expected size of 117 and 87 kD, respectively (*). No radiolabeling of MBP is observed.