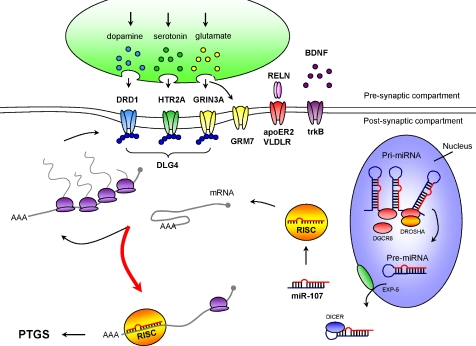
Figure 5.
Model for miRNA-associated dysregulation of synaptic structure and function in schizophrenia. The microprocessor activity is elevated in cortical nuclei as a consequence of a schizophrenia-associated increase in DGCR8 expression. The increase in pri-miRNA processing results in an increase in pre-miRNAs, which are exported from the nucleus and processed without delay by Exportin-5 (XPO5) and Dicer, respectively. Mature miRNAs are recruited into the RNA-induced silencing complex (RISC) and associate with the 3′-UTR of their target transcripts encoding synaptic components (among other proteins), such as neurotropins/ligands (BDNF, Reelin), neurotransmitter receptors (GRM7, GRIN3A, HTR2A, DRD1) and structural components of the post-synaptic density (DLG4). This association reduces the stability of the transcript and reduces its ability to undergo translation. Inappropriate levels of mature miRNA and gene silencing (red arrow) result in the reduction of synaptic proteins and consequently a loss of synaptic structure and function.