Abstract
Voltage-gated K+ (Kv) channels regulate diverse neuronal properties including action potential threshold, amplitude, and duration, frequency of firing, neurotransmitter release, and resting membrane potential. In axons, Kv channels are clustered at a variety of functionally important sites including axon initial segments, juxtaparanodes of myelinated axons, nodes of Ranvier, and cerebellar basket cell terminals. These channels are part of larger protein complexes that include cell adhesion molecules and scaffolding proteins. These interacting proteins play important roles in recruiting K+ channels to distinct axonal domains. Here, I review the composition, functions, and mechanism of localization of these K+ channel complexes in axons.
Keywords: action potential, juxtaparanode, cell adhesion molecule, MAGUK, K+ channel
INTRODUCTION
Neurons are morphologically complex cells with two major structural and functional domains: the somatodendritic and axonal domains. The somatodendritic domain receives and integrates synaptic input, while the axonal domain is responsible for initiation and propagation of the action potential. The excitable properties of each of these domains depends not only on the kinds of ion channels expressed in the plasma membrane, but also on the location of the channels and receptors distributed throughout the plasma membrane. For example, ligand-gated ion channels are strategically located and enriched in membrane domains opposite the presynaptic terminal where neurotransmitter is released. Among the many different voltage-gated ion channels expressed in the nervous system, the voltage-gated K+ (Kv) channels have been a favorite of neurobiologists due to their highly restricted locations in axons, their important contributions to neuronal excitability, their diverse mechanisms of clustering, and their experimental tractability as compared to much larger axonal ion channels (e.g. Na+ and Ca2+ channels). Furthermore, the importance of these channels is reflected in the fact that mutations or diseases that disrupt clustering, localization, or composition of axonal Kv channel complexes compromises nervous system function, leading to conduction block, episodic ataxia, and/or epilepsies [11, 14, 29, 31, 32].
In axons, four main Kv channel protein complexes have been described. These consist of Kv1 (Kv1.1, Kv1.2, and Kv1.4), Kv2 (Kv2.1 and Kv2.2), Kv3.1b, or Kv7 (Kv7.2 and Kv7.3, but also referred to as KCNQ2 and KCNQ3, respectively (in this review I will refer to these channels as KCNQ2 and KCNQ3)) channel subunits [52]. Here, I will discuss what we know about the locations, functions, molecular compositions, and mechanisms of clustering for each of these different Kv channel complexes in axons.
KCNQ2/3 K+ channels
KCNQ K+ channels are broadly expressed, and mutations in these channels lead to a variety of channelopathies including epilepsy, deafness, and disrupted cardiac function[23]. In neurons, KCNQ2 and KCNQ3 channels have been reported at axon initial segments (AIS; Fig. 1A) and nodes of Ranvier (Fig. 1B) [8, 36]. Physiologically, these channels underlie the so-called M-current, and play essential roles in regulating neuronal and axonal excitability through their actions at nodes and initial segments [3, 47]. Mutations in KCNQ2 and KCNQ3 lead to neonatal epilepsies, including benign neonatal familial convulsions (BNFC).
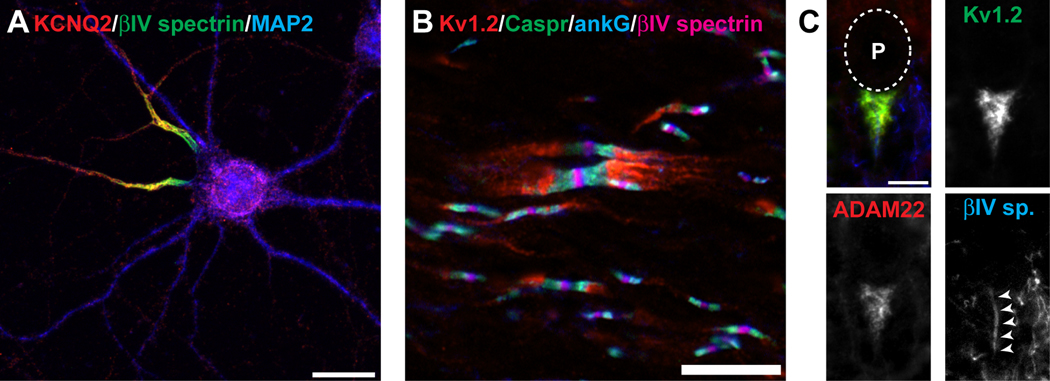
Figure 1. Kv channels are clustered at a many different axonal locations.
A, KCNQ2 K+ channels (red) are clustered at axon initial segments where they colocalize with the AIS-restricted cytoskeletal scaffolding protein βIV spectrin (gree). The microtubule associated protein 2 (MAP2) defines the somatodendritic domain. B, Kv1 channels (red) are clustered at juxtaparanodes beneath the myelin sheath and on each side of nodes of Ranvier. Kv1 channels are excluded from paranodal regions labeled by Caspr (green), and nodes of Ranvier labeled by ankG (blue) and βIV spectrin (magenta). C, Basket cell terminals in the cerebellum are highly enriched in Kv1.2 (green) and ADAM22 (red), and envelope the AIS (labeled by βIV spectrin, blue). The location of the Purkinje neuron cell body (P) is indicated by the dotted line. Scale bars: A, 20 µm; B, C, 10 µm.
In axons, the only known binding partner for KCNQ2 and KCNQ3 is the large cytoskeletal scaffolding protein ankyrinG (ankG) [36], and KCNQ2/3 colocalizes with ankG at both the AIS and nodes. AnkG is highly enriched at nodes of Ranvier and AIS and plays essential roles in both the initial assembly of the AIS and long-term maintenance of neuronal polarity, i.e. the distinction between axonal and somatodendritic domains. However, the mechanism responsible for clustering ankG at the AIS remain unknown (Fig. 2). AnkG binds directly to a variety of Na+ channels (including Nav1.1, Nav1.2 and Nav1.6) which underlie the initiation of the action potential, cell adhesion molecules (NrCAM and Neurofascin-186), and other cytoskeletal and scaffolding proteins (βIV spectrin) enriched at the AIS [35]. Indeed, experiments to silence expression of ankG demonstrate that its loss results in failure to recruit all other AIS proteins, including KCNQ2 and KCNQ3 [22, 36].
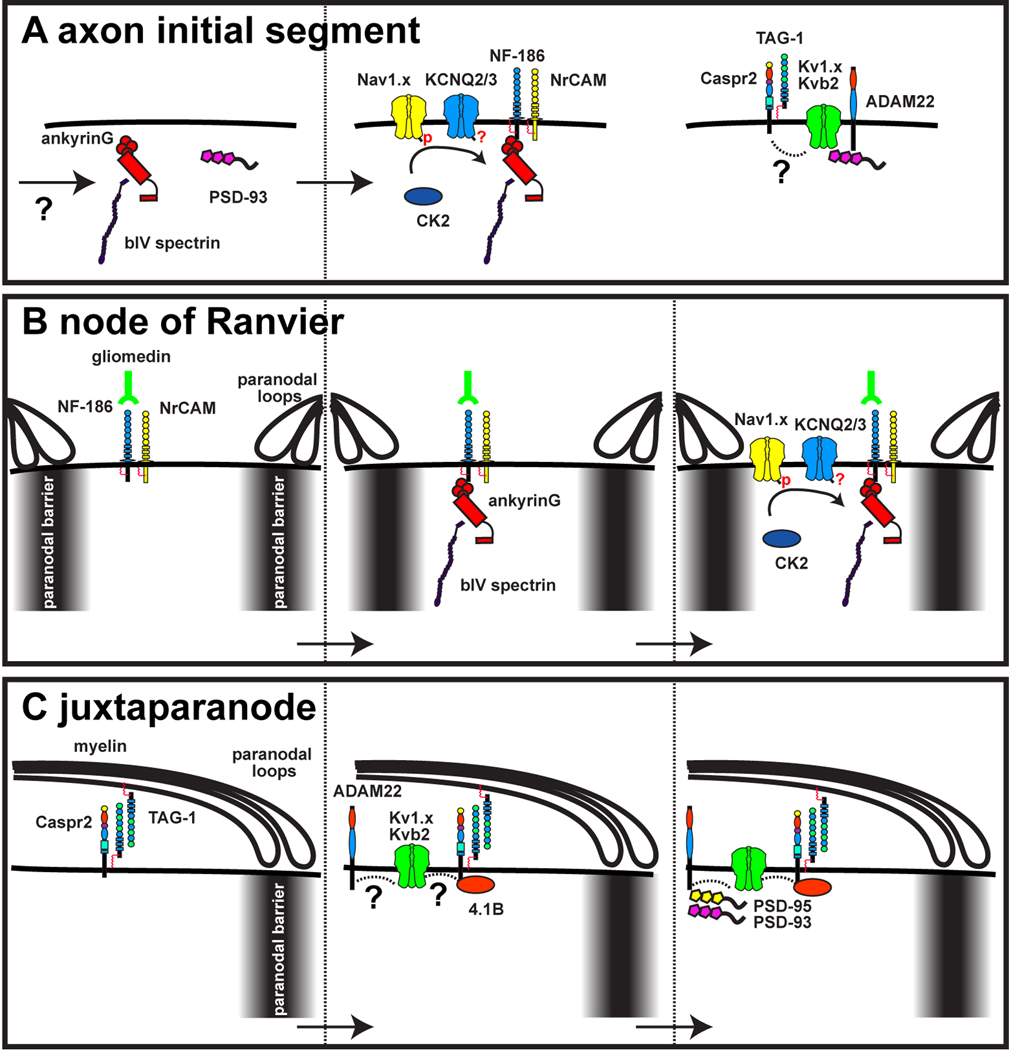
Figure 2. Mechanisms of channel localization in axons.
A, The scaffolds ankyrinG and PSD-93 are recruited to the AIS through unknown mechanisms. They subsequently recruit cell adhesion molecules and ion channels. Whether phosphorylation promotes the interaction between ankG and KCNQ channels is unknown. The link between Caspr2 and the Kv1 channel protein complex is also unknown. B, Axonal cell adhesion molecules are restricted to nodes of Ranvier through clustering by Schwann cell-derived gliomedin, and by restriction between a paranodal barrier. AnkG then attaches to these cell adhesion molecules, and functions as at the AIS to recruit ion channels. Thus, at nodes, myelinating glia direct the clustering of channels. C, At the juxtaparanode the cell adhesion molecules Caspr2 and TAG-1 are thought to initiate the assembly of the Kv1 channel protein complex. However, the interactions between Caspr2 and Kv1 channels remain unknown. ADAM22 recruits PSD-95 and PSD-93. A paranodal barrier also restricts the lateral diffusion of Kv1 channels in the axonal membrane.
The clustering of Na+ channels at the AIS was shown to depend on an AIS targeting motif found in the II–III linker of Na+ channels[13, 27]. Pan et al. [36], noticed that a similar motif is located near the C-terminus of KCNQ2 and KCNQ3 channels. They then demonstrated that this sequence does in fact mediate the channel’s interaction with ankG. In a remarkable follow-up paper, Hill et al. [17], further analyzed this AIS targeting sequence in both Na+ and KCNQ channels and found that the sequence evolved first in basal chordates to permit Na+ channel clustering and retention at the AIS. They then demonstrated that it wasn’t until much later in evolution (about the time myelin evolved), that the KCNQ2/3 channels acquired the AIS targeting motif. They were able to show that this sequence evolved independently, providing the first known example of convergent molecular evolution. Furthermore, their results strongly suggested that nodes of Ranvier are evolutionary derivatives of the AIS, and the requirements of Na+ channel clustering in axons likely drove the evolution of KCNQ2/3 K+ channel clustering. Mutation of the ankG-interacting motif in KCNQ2 and KCNQ3 blocks the ability of these subunits to become restricted to the AIS [43].
Intriguingly, one recent paper demonstrated that the binding of Nav channels to ankG is strongly facitiliated by Casein Kinase 2 (CK2) – dependent phosphorylation of serine residues within the AIS targeting motif[2] (Fig. 2). Since KCNQ K+ channels have a similar motif, it will be interesting to determine if localization of these channels can also be regulated by CK2.
At the AIS, the initial events responsible for ankG clustering remain unknown. However, at nodes of Ranvier it appears that two different kinds of neuron-glia interactions can initiate clustering of ankG (Fig. 2B). In the peripheral nervous system, Schwann cells secrete a protein called gliomedin that is incorporated into the extracellular matrix and binds to the axonal cell adhesion molecule neurofascin (NF) -186[10]. NF-186, in turn, is a binding partner for ankG and functions as a nucleation site for the recruitment of ankG and the subsequent clustering of Na+ and KCNQ2/3 K+ channels[4, 9]. Intriguingly, experiments also indicate that a second, overlapping mechanism exists to facilitate node of Ranvier assembly, one which depends on interactions between the myelin sheath and the axon at paranodal junctions (Fig. 1B) [12, 42, 58]. These interactions create a barrier that limits the lateral diffusion of ion channels and other nodal proteins. Although the molecular details for how this happens are lacking, ankG appears to be critical in both situations. However, the importance of ankG for KCNQ2/3 channel clustering at nodes has only been inferred from in vitro experiments examining Na+ channel clustering; loss of ankG from neurons in myelinating co-cultures blocks the clustering of Na+ channels[9]. Since the interaction of Na+ channels and KCNQ2/3 K+ channels with ankG depends on the same AIS targeting motif, it is likely that nodal localization of KCNQ2/3 channels is also mediated by ankG.
Kv3.1b channels
Kv3 channels contribute to the rapid spiking behavior of many neurons[44]. Among the Kv3 channels, Kv3.1b, a unique splice variant of the Kv3.1 gene, has been reported at a subset of CNS and PNS nodes of Ranvier [5] with greater extent of expression in the CNS. Intriguingly, although biochemical analyses showed that it can be co-immunopreciptated with ankG, Kv3.1b was not detected at the AIS. Indeed, Kv3.1b is the only known nodal ion channel that is not also found at the AIS (in direct contrast, Kv1 channels are detected at the AIS, but not nodes; see below). The reasons for this disparity are unknown. Subsequent studies to investigate the mechanism of Kv3.1b trafficking to axons showed that axonal targeting depends on a motif in the C-terminus of the channel that cooperates both with the T1-domain of the channel and ankG[56], which is consistent with the earlier biochemical studies. Nevertheless, the function of Kv3.1b at nodes of Ranvier remains unknown.
Kv2 channels
Kv2 channels are delayed rectifiers thought to mainly regulate membrane excitability in somatodendritic domains. However, recent studies have shown that Kv2 channels can also be enriched at the AIS through as yet unknown mechanisms, although Kv2 channels have not been described at nodes or any other axonal subdomain. In particular, Kv2.1 was reported at the AIS of both cultured hippocampal neurons and cortical neurons in vivo[46], and Kv2.2 was described at the AIS of neurons found in the medial nucleus of the trapezoid body (MNTB) where it supports high action potential firing frequencies [24]. Measurements of Kv2.1 diffusion rates showed that it is highly mobile in the AIS, a result that is contrary to other studies showing that both lipids and proteins have reduced diffusion rates and can be stable over days and even weeks at the AIS[16, 55]. This observation suggests that Kv2 channel accumulation may not depend directly on ankG, or that other more dynamic mechanisms exist for its high degree of mobility in the AIS.
Kv1 K+ channels
High-density clusters of Kv1 channels in axons were first shown at the juxtaparanodes of myelinated axons and the basket cell terminals (BCTs) of cerebellar pinceau (Figs. 1B and 1C, respectively) [54]. More recently, Kv1 channel complexes have also been shown to be enriched at a third site in axons, the AIS [15, 21, 26, 33]. In each of these different domains, axonal Kv1 channels form macromolecular protein complexes with a variety of cell adhesion molecules and cytoskeletal scaffolding proteins.
Basket cell terminals
The subunits Kv1.1 and Kv1.2 have been shown to be highly enriched at BCTs [54]. Here, they form a specialized contact site with the AIS of the Purkinje neuron (Fig. 1C) and regulate GABAergic inhibition of the Purkinje neuron efferent axon[57]. BCT Kv1.1/1.2 channels associate with many proteins (Table I), including their accessory β-subunit Kvβ2, which has been shown to promote surface expression of the channel[48]. The membrane associated guanylate kinase (MAGUK) PSD-95 is also highly enriched at BCTs. PSD-95 consists of 3 PDZ domains, each of which can interact with the C-terminal PDZ binding motif found in Kv1 channels[25]. The interaction with PSD-95 was originally thought to be a mechanism for clustering of these channels at BCTs and juxtaparanodes, but subsequent studies of mice lacking PSD-95 at these sites showed that Kv1 channels could still be clustered[41]. Finally, one other Kv1 channel interacting partner was recently reported to be present at BCTs: ADAM22 (a disintegrin and metalloprotease 22)[34]. ADAM22 was originally identified as a Kv1 channel binding partner by analyzing Kv1.2-subunit brain immunoprecipitations using mass-spectrometry. ADAM22 was then demonstrated at BCTs and other axonal sites where Kv1 channels are clustered. ADAM22 is a transmembrane protein that belongs to a large family of proteases, although ADAM22 itself has no catalytic activity. Intriguingly, ADAM22 also harbors a PDZ binding motif that results in strong binding to PSD-95. In contrast to K+ channels, loss of PSD-95 from BCTs completely eliminates the clustering of ADAM22 at these sites. Nevertheless, the function of ADAM22 at BCTs is unknown since ADAM22 knockout mice die at P12, before the assembly of the BCT[34, 45].
Table 1.
Kv channel protein complexes and the sites where they are clustered. ●= detected, ○ = not detected.
| Axon Initial Segment | Juxtaparanode | Node of Ranvier | Basket Cell Terminal | |
|---|---|---|---|---|
| KCNQ2/3 | ● | ○ | ● | ○ |
| Kv3.1b | ○ | ○ | ● | ○ |
| Kv2.1/Kv2.2 | ● | ○ | ○ | ○ |
| ankyrinG | ● | ○ | ● | ○ |
| Kv1.x | ● | ● | ○ | ● |
| Kvβ2 | ● | ● | ○ | ● |
| Caspr2 | ● | ● | ○ | ○ |
| TAG-1 | ● | ● | ○ | ○ |
| PSD-93 | ● | ● | ○ | ○ |
| PSD-95 | ○ | ● | ○ | ● |
| 4.1B | ○ | ● | ○ | ○ |
| ADAM22 | ● | ● | ○ | ● |
Juxtaparanodes
The Kv1 channel subunits Kv1.1, Kv1.2, and Kv1.4 have all been reported at juxtaparanodes of myelinated axons where they are thought to modulate action potential propagation and dampen repetitive firing of injured and developing myelinated axons[40]. Indeed, the location of these channels beneath the myelin sheath, where they are apparently electrically isolated, has caused no small degree of controversy surrounding their functions in normal, mature myelinated fibers. However, their ‘isolation’ has been called into questions since in some small diameter myelinated axons of the central nervous system, these channels can modulate action potential properties such that pharmacological block of these channels results in dramatic prolongation of the action potential[6, 7].
Like the BCT, juxtaparanodal K+ channels are part of a larger protein complex (Table I). In addition to Kvβ-subunits, juxtaparanodes are also enriched in the cell adhesion molecules (CAMs) Caspr2, TAG-1, and ADAM22, the MAGUKs PSD-93 and PSD-95, and the cytoskeletal scaffold 4.1B[19, 37, 38, 50]. Studies of knockout mice for each of these different proteins has revealed important information about the roles each protein plays in assembling juxtaparanodal Kv1 channel complexes.
Knockout of Kvβ2-subunits, the main Kvβ subunit reported at juxtaparanodes, does not affect the clustering of juxtaparanodal Kv1 channels[30]. Similarly, the MAGUKs PSD-93 and PSD-95 are both dispensible for Kv1 channel clustering. Even double-knockouts, lacking both PSD-93 and PSD-95 still have clustered channels, indicating that in single knock-outs these MAGUKs do not compensate for one another [19]. Knockout of ADAM22 has no effect on clustering of Kv1 channels at juxtaparanodes, but in contrast to BCTs completely blocks the recruitment of the MAGUKs PSD-93 and PSD-95[34] (Fig. 2C). Thus, ADAM22 is responsible for MAGUK clustering at juxtaparanodes, but not for Kv1 channel clustering.
Instead, knock-outs of the cell-adhesion molecules Caspr2 and TAG-1 dramatically impair juxtaparanodal clustering of Kv1 channel protein complexes[39, 51]. Caspr2 and TAG-1 are thought to form a heterodimer in the axolemma that interacts in trans with TAG-1 expressed on the inner surface of the myelin sheath (Fig. 2C). While loss of Caspr2 and TAG-1 disrupt Kv1 channel clustering in peripheral axons, about one-third of juxtparanodes in the CNS of Caspr2-null mice still have detectable juxtaparanodal Kv1 channels[34], suggesting that additional mechanisms exist that can also facilitate channel clustering. Loss of Caspr2 or TAG-1 also impairs the clustering of MAGUKs and ADAM22[19, 34], but not protein 4.1B[18], suggesting that 4.1B may also contribute to either the clustering or retention of Kv1 channel complexes. Consistent with this idea, Caspr2 has a 4.1B binding-domain, which is necessary for its accumulation at juxtaparanodes [18]. The importance of the interaction with 4.1B for Caspr2 clustering was explicitly demonstrated in Caspr2-null mice that expressed a transgene containing the extracellular domain of Caspr2, but lacking the 4.1B binding domain. In these mice, the Caspr2 transgene, Kv1 channels, and TAG-1 all failed to cluster at juxtaparanodes. Consistent with these results, loss of 4.1B also impairs assembly of the Kv1 channel-containing complexes at juxtaparanodes. It will be of particular interest to determine if the loss of 4.1B affects the initial recruitment of the channel complex, or if its function is primarily to stabilize the protein complex and retain it at juxtaparanodes.
Axon initial segments
As described above, in myelinated axons Kv1 channels are excluded from nodal regions that instead are enriched in Na+ channels and KCNQ2/3 K+ channels. Intriguingly, Kv1 channels, Caspr2, TAG-1, ADAM22, and PSD-93 are also found colocalized with AIS ion channels, but only in the distal part of the AIS where Nav1.6 Na+ channels are prominently found [20, 28, 34, 53]. In contrast to both juxtaparanodes and BCTs, AIS Kv1 channel clustering was reported to depend on PSD-93 rather than CAMs [33] (Fig. 2A). This result was based on the use of shRNA knockdown of PSD-93 in cultured hippocampal neurons, a rarefied culture model that has been used to study the molecular mechanisms underlying many neuronal properties. However, in more recent experiments, new antigen-retrieval methods for visualizing Kv1 channels in brain [28] prompted a re-examination AIS Kv1 channels in PSD-93 deficient mouse cortex. In contrast to the in vitro results, loss of PSD-93 did not impair clustering of Kv1 channels at the AIS[34]. This discrepancy between in vitro and in vivo results may reflect real differences between acute knockdown of PSD-93 versus chronic loss of the protein in PSD-93 deficient mice which could permit compensation by other unknown mechanisms. Alternatively, this difference may also underscore the point that in vitro culture models may be much more sensitive to experimental manipulation than in vivo, where other compensatory mechanisms may exist for channel clustering. It is interesting to note that mutations in the Kv1 channel interacting protein Caspr2 leads to a variety of CNS abnormalities including epilepsy, mental retardation, Tourette syndrome, and Autism Spectrum Disorder [1, 49, 59]. Whether these diseases are a consequences of disrupted Caspr2 at the AIS, or other axonal locations remains unknown.
Future directions
Although work on axonal Kv channels has revealed tremendous insights into the mechanisms of channel function and recruitment to distinct axonal domains, much remains unknown. In the case of Kv1 channels, we do not fully understand the function of these channels at juxtaparanodes. In addition, experiments to determine mechanisms of targeting have shown that other, Caspr2- or PSD-93-independent mechanisms of targeting to juxtaparanodes and AIS, respectively, must exist. These remain to be identified. Furthermore, the function of the MAGUKs PSD-93 and PSD-95 at juxtaparanodes and BCTs is completely unknown, although we can exclude a role in Kv1 channel complex assembly. Similarly, many questions remain about the functions and protein complexes that include Kv2 channels, Kv3.1b, and KCNQ2/3 K+ channels. For example, although we assume KCNQ2/3 channel targeting to nodes is ankG dependent, this remains to be formally demonstrated. Furthermore, the functional properties of these channels can be highly modified by phosphorylation, phospholipids, and interacting accessory subunits. It will be important to determine if AIS or nodal KCNQ2/3 channels are modulated in any of these ways. Similarly, it is not clear why Kv3.1b bypasses the AIS and is clustered only at nodes of Ranvier, especially if this localization depends directly on ankG. In contrast, it will be interesting to determine if Kv2 channel localization at the AIS depends on ankG, and if so, how it retains its high degree of mobility in the AIS membrane. Finally, it will be important to determine the functions of the many different Kv1 channel interacting proteins like ADAM22, Caspr2, TAG-1 and MAGUKs. It is not difficult to imagine that they may directly modulate channel properties depending on the cellular context and location, and in ways that we have not yet imagined. Given the many questions that have yet to be answered, Kv channels will remain excellent models to uncover the mechanisms for how ion channel complexes contribute to axonal physiology, and how they are assembled, trafficked to, clustered, and retained at distinct cellular locations.
Acknowledgements
Supported by NIH grant NS044916. MNR is a Harry Weaver Neuroscience Scholar of the National Multiple Sclerosis Society.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Belloso JM, Bache I, Guitart M, Caballin MR, Halgren C, Kirchhoff M, Ropers HH, Tommerup N, Tumer Z. Disruption of the CNTNAP2 gene in a t(7;15) translocation family without symptoms of Gilles de la Tourette syndrome. Eur J Hum Genet. 2007;15:711–713. doi: 10.1038/sj.ejhg.5201824. [DOI] [PubMed] [Google Scholar]
- 2.Bréchet A, Fache MP, Brachet A, Ferracci G, Baude A, Irondelle M, Pereira S, Leterrier C, Dargent B. Protein kinase CK2 contributes to the organization of sodium channels in axonal membranes by regulating their interaction with ankyrin G. J Cell Biol. 2008;183:1101–1114. doi: 10.1083/jcb.200805169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown DA, Passmore GM. Neural KCNQ (Kv7) channels. Br J Pharmacol. 2009;156:1185–1195. doi: 10.1111/j.1476-5381.2009.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis JQ, Lambert S, Bennett V. Molecular composition of the node of Ranvier: identification of ankyrin-binding cell adhesion molecules neurofascin (mucin+/third FNIII domain-) and NrCAM at nodal axon segments. J Cell Biol. 1996;135:1355–1367. doi: 10.1083/jcb.135.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devaux J, Alcaraz G, Grinspan J, Bennett V, Joho R, Crest M, Scherer SS. Kv3.1b is a novel component of CNS nodes. J Neurosci. 2003;23:4509–4518. doi: 10.1523/JNEUROSCI.23-11-04509.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devaux J, Gola M, Jacquet G, Crest M. Effects of K+ channel blockers on developing rat myelinated CNS axons: identification of four types of K+ channels. J Neurophysiol. 2002;87:1376–1385. doi: 10.1152/jn.00646.2001. [DOI] [PubMed] [Google Scholar]
- 7.Devaux J, Gow A. Tight junctions potentiate the insulative properties of small CNS myelinated axons. J Cell Biol. 2008;183:909–921. doi: 10.1083/jcb.200808034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devaux JJ, Kleopa KA, Cooper EC, Scherer SS. KCNQ2 Is a Nodal K+ Channel. J Neurosci. 2004;24:1236–1244. doi: 10.1523/JNEUROSCI.4512-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dzhashiashvili Y, Zhang Y, Galinska J, Lam I, Grumet M, Salzer JL. Nodes of Ranvier and axon initial segments are ankyrin G-dependent domains that assemble by distinct mechanisms. J Cell Biol. 2007;177:857–870. doi: 10.1083/jcb.200612012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eshed Y, Feinberg K, Poliak S, Sabanay H, Sarig-Nadir O, Spiegel I, Bermingham JR, Jr, Peles E. Gliomedin mediates schwann cell-axon interaction and the molecular assembly of the nodes of ranvier. Neuron. 2005;47:215–229. doi: 10.1016/j.neuron.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 11.Eunson LH, Rea R, Zuberi SM, Youroukos S, Panayiotopoulos CP, Liguori R, Avoni P, McWilliam RC, Stephenson JB, Hanna MG, Kullmann DM, Spauschus A. Clinical, genetic, and expression studies of mutations in the potassium channel gene KCNA1 reveal new phenotypic variability. Ann Neurol. 2000;48:647–656. [PubMed] [Google Scholar]
- 12.Feinberg K, Eshed-Eisenbach Y, Frechter S, Amor V, Salomon D, Sabanay H, Dupree JL, Grumet M, Brophy PJ, Shrager P, Peles E. A glial signal consisting of gliomedin and NrCAM clusters axonal Na+ channels during the formation of nodes of Ranvier. Neuron. 2010;65:490–502. doi: 10.1016/j.neuron.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garrido JJ, Giraud P, Carlier E, Fernandes F, Moussif A, Fache MP, Debanne D, Dargent B. A targeting motif involved in sodium channel clustering at the axonal initial segment. Science. 2003;300:2091–2094. doi: 10.1126/science.1085167. [DOI] [PubMed] [Google Scholar]
- 14.Glasscock E, Yoo JW, Chen TT, Klassen TL, Noebels JL. Kv1.1 potassium channel deficiency reveals brain-driven cardiac dysfunction as a candidate mechanism for sudden unexplained death in epilepsy. J Neurosci. 2010;30:5167–5175. doi: 10.1523/JNEUROSCI.5591-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldberg EM, Clark BD, Zagha E, Nahmani M, Erisir A, Rudy B. K+ channels at the axon initial segment dampen near-threshold excitability of neocortical fast-spiking GABAergic interneurons. Neuron. 2008;58:387–400. doi: 10.1016/j.neuron.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hedstrom KL, Ogawa Y, Rasband MN. AnkyrinG is required for maintenance of the axon initial segment and neuronal polarity. J Cell Biol. 2008;183:635–640. doi: 10.1083/jcb.200806112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill AS, Nishino A, Nakajo K, Zhang G, Fineman JR, Selzer ME, Okamura Y, Cooper EC. Ion channel clustering at the axon initial segment and node of ranvier evolved sequentially in early chordates. PLoS Genet. 2008;4:e1000317. doi: 10.1371/journal.pgen.1000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horresh I, Bar V, Kissil JL, Peles E. Organization of myelinated axons by Caspr and Caspr2 requires the cytoskeletal adapter protein 4.1B. J Neurosci. 2010;30:2480–2489. doi: 10.1523/JNEUROSCI.5225-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horresh I, Poliak S, Grant S, Bredt D, Rasband MN, Peles E. Multiple molecular interactions determine the clustering of Caspr2 and Kv1 channels in myelinated axons. J Neurosci. 2008;28:14213–14222. doi: 10.1523/JNEUROSCI.3398-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu W, Tian C, Li T, Yang M, Hou H, Shu Y. Distinct contributions of Na(v)1.6 and Na(v)1.2 in action potential initiation and backpropagation. Nat Neurosci. 2009;12:996–1002. doi: 10.1038/nn.2359. [DOI] [PubMed] [Google Scholar]
- 21.Inda MC, Defelipe J, Munoz A. Voltage-gated ion channels in the axon initial segment of human cortical pyramidal cells and their relationship with chandelier cells. Proc Natl Acad Sci U S A. 2006 doi: 10.1073/pnas.0511197103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenkins SM, Bennett V. Ankyrin-G coordinates assembly of the spectrin-based membrane skeleton, voltage-gated sodium channels, and L1 CAMs at Purkinje neuron initial segments. J Cell Biol. 2001;155:739–746. doi: 10.1083/jcb.200109026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jentsch TJ. Neuronal KCNQ potassium channels: physiology and role in disease. Nat Rev Neurosci. 2000;1:21–30. doi: 10.1038/35036198. [DOI] [PubMed] [Google Scholar]
- 24.Johnston J, Griffin SJ, Baker C, Skrzypiec A, Chernova T, Forsythe ID. Initial segment Kv2.2 channels mediate a slow delayed rectifier and maintain high frequency action potential firing in MNTB neurones. J Physiol. 2008 doi: 10.1113/jphysiol.2008.153734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim E, Niethammer M, Rothschild A, Jan YN, Sheng M. Clustering of Shaker-type K+ channels by interaction with a family of membrane-associated guanylate kinases. Nature. 1995;378:85–88. doi: 10.1038/378085a0. [DOI] [PubMed] [Google Scholar]
- 26.Kole MH, Letzkus JJ, Stuart GJ. Axon initial segment Kv1 channels control axonal action potential waveform and synaptic efficacy. Neuron. 2007;55:633–647. doi: 10.1016/j.neuron.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 27.Lemaillet G, Walker B, Lambert S. Identification of a conserved ankyrin-binding motif in the family of sodium channel alpha subunits. J Biol Chem. 2003;278:27333–27339. doi: 10.1074/jbc.M303327200. [DOI] [PubMed] [Google Scholar]
- 28.Lorincz A, Nusser Z. Cell-type-dependent molecular composition of the axon initial segment. J Neurosci. 2008;28:14329–14340. doi: 10.1523/JNEUROSCI.4833-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manganas LN, Akhtar S, Antonucci DE, Campomanes CR, Dolly JO, Trimmer JS. Episodic ataxia type-1 mutations in the Kv1.1 potassium channel display distinct folding and intracellular trafficking properties. J Biol Chem. 2001;276:49427–49434. doi: 10.1074/jbc.M109325200. [DOI] [PubMed] [Google Scholar]
- 30.McCormack K, Connor JX, Zhou L, Ho LL, Ganetzky B, Chiu SY, Messing A. Genetic analysis of the mammalian K+ channel beta subunit Kvbeta 2 (Kcnab2) J Biol Chem. 2002;277:13219–13228. doi: 10.1074/jbc.M111465200. [DOI] [PubMed] [Google Scholar]
- 31.Misonou H. Homeostatic regulation of neuronal excitability by K(+) channels in normal and diseased brains. Neuroscientist. 2010;16:51–64. doi: 10.1177/1073858409341085. [DOI] [PubMed] [Google Scholar]
- 32.Nashmi R, Fehlings MG. Mechanisms of axonal dysfunction after spinal cord injury: with an emphasis on the role of voltage-gated potassium channels. Brain Res Brain Res Rev. 2001;38:165–191. doi: 10.1016/s0165-0173(01)00134-5. [DOI] [PubMed] [Google Scholar]
- 33.Ogawa Y, Horresh I, Trimmer JS, Bredt DS, Peles E, Rasband MN. Postsynaptic density-93 clusters Kv1 channels at axon initial segments independently of Caspr2. J Neurosci. 2008;28:5731–5739. doi: 10.1523/JNEUROSCI.4431-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogawa Y, Oses-Prieto J, Kim MY, Horresh I, Peles E, Burlingame AL, Trimmer JS, Meijer D, Rasband MN. ADAM22, a Kv1 channel-interacting protein, recruits membrane-associated guanylate kinases to juxtaparanodes of myelinated axons. J Neurosci. 2010;30:1038–1048. doi: 10.1523/JNEUROSCI.4661-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogawa Y, Rasband MN. The functional organization and assembly of the axon initial segment. Curr Opin Neurobiol. 2008;18:307–313. doi: 10.1016/j.conb.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 36.Pan Z, Kao T, Horvath Z, Lemos J, Sul J-Y, Cranstoun SD, Bennett MV, Scherer SS, Cooper EC. A common ankyrin-G-based mechanism retains KCNQ and Nav channels at electrically active domains of the axon. J Neurosci. 2006;26:2599–2613. doi: 10.1523/JNEUROSCI.4314-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poliak S, Gollan L, Martinez R, Custer A, Einheber S, Salzer JL, Trimmer JS, Shrager P, Peles E. Caspr2, a new member of the neurexin superfamily, is localized at the juxtaparanodes of myelinated axons and associates with K+ channels. Neuron. 1999;24:1037–1047. doi: 10.1016/s0896-6273(00)81049-1. [DOI] [PubMed] [Google Scholar]
- 38.Poliak S, Gollan L, Salomon D, Berglund EO, Ohara R, Ranscht B, Peles E. Localization of Caspr2 in myelinated nerves depends on axon-glia interactions and the generation of barriers along the axon. J Neurosci. 2001;21:7568–7575. doi: 10.1523/JNEUROSCI.21-19-07568.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poliak S, Salomon D, Elhanany H, Sabanay H, Kiernan B, Pevny L, Stewart CL, Xu X, Chiu SY, Shrager P, Furley AJ, Peles E. Juxtaparanodal clustering of Shaker-like K+ channels in myelinated axons depends on Caspr2 and TAG-1. J Cell Biol. 2003;162:1149–1160. doi: 10.1083/jcb.200305018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rasband MN. It's 'juxta' potassium channel. J Neurosci Res. 2004;76:749–757. doi: 10.1002/jnr.20073. [DOI] [PubMed] [Google Scholar]
- 41.Rasband MN, Park EW, Zhen D, Arbuckle MI, Poliak S, Peles E, Grant SGN, Trimmer JS. Clustering of neuronal potassium channels is independent of their interaction with PSD-95. J Cell Biol. 2002;159:663–672. doi: 10.1083/jcb.200206024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rasband MN, Peles E, Trimmer JS, Levinson SR, Lux SE, Shrager P. Dependence of nodal sodium channel clustering on paranodal axoglial contact in the developing CNS. J Neurosci. 1999;19:7516–7528. doi: 10.1523/JNEUROSCI.19-17-07516.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rasmussen HB, Frokjaer-Jensen C, Jensen CS, Jensen HS, Jorgensen NK, Misonou H, Trimmer JS, Olesen SP, Schmitt N. Requirement of subunit co-assembly and ankyrin-G for M-channel localization at the axon initial segment. J Cell Sci. 2007;120:953–963. doi: 10.1242/jcs.03396. [DOI] [PubMed] [Google Scholar]
- 44.Rudy B, McBain CJ. Kv3 channels: voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci. 2001;24:517–526. doi: 10.1016/s0166-2236(00)01892-0. [DOI] [PubMed] [Google Scholar]
- 45.Sagane K, Hayakawa K, Kai J, Hirohashi T, Takahashi E, Miyamoto N, Ino M, Oki T, Yamazaki K, Nagasu T. Ataxia and peripheral nerve hypomyelination in ADAM22-deficient mice. BMC Neurosci. 2005;6:33. doi: 10.1186/1471-2202-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sarmiere PD, Weigle CM, Tamkun MM. The Kv2.1 K+ channel targets to the axon initial segment of hippocampal and cortical neurons in culture and in situ. BMC Neurosci. 2008;9:112. doi: 10.1186/1471-2202-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwarz JR, Glassmeier G, Cooper EC, Kao TC, Nodera H, Tabuena D, Kaji R, Bostock H. KCNQ channels mediate IKs, a slow K+ current regulating excitability in the rat node of Ranvier. J Physiol. 2006;573:17–34. doi: 10.1113/jphysiol.2006.106815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi G, Nakahira K, Hammond S, Rhodes KJ, Schechter LE, Trimmer JS. Beta subunits promote K+ channel surface expression through effects early in biosynthesis. Neuron. 1996;16:843–852. doi: 10.1016/s0896-6273(00)80104-x. [DOI] [PubMed] [Google Scholar]
- 49.Strauss KA, Puffenberger EG, Huentelman MJ, Gottlieb S, Dobrin SE, Parod JM, Stephan DA, Morton DH. Recessive symptomatic focal epilepsy and mutant contactin-associated protein-like 2. N Engl J Med. 2006;354:1370–1377. doi: 10.1056/NEJMoa052773. [DOI] [PubMed] [Google Scholar]
- 50.Traka M, Dupree JL, Popko B, Karagogeos D. The neuronal adhesion protein TAG-1 is expressed by Schwann cells and oligodendrocytes and is localized to the juxtaparanodal region of myelinated fibers. J Neurosci. 2002;22:3016–3024. doi: 10.1523/JNEUROSCI.22-08-03016.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Traka M, Goutebroze L, Denisenko N, Bessa M, Nifli A, Havaki S, Iwakura Y, Fukamauchi F, Watanabe K, Soliven B, Girault JA, Karagogeos D. Association of TAG-1 with Caspr2 is essential for the molecular organization of juxtaparanodal regions of myelinated fibers. J Cell Biol. 2003;162:1161–1172. doi: 10.1083/jcb.200305078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trimmer JS, Rhodes KJ. Localization of voltage-gated ion channels in mammalian brain. Annu Rev Physiol. 2004;66:477–519. doi: 10.1146/annurev.physiol.66.032102.113328. [DOI] [PubMed] [Google Scholar]
- 53.Van Wart A, Trimmer JS, Matthews G. Polarized distribution of ion channels within microdomains of the axon initial segment. J Comp Neurol. 2007;500:339–352. doi: 10.1002/cne.21173. [DOI] [PubMed] [Google Scholar]
- 54.Wang H, Kunkel DD, Martin TM, Schwartzkroin PA, Tempel BL. Heteromultimeric K+ channels in terminal and juxtaparanodal regions of neurons. Nature. 1993;365:75–79. doi: 10.1038/365075a0. [DOI] [PubMed] [Google Scholar]
- 55.Winckler B, Forscher P, Mellman I. A diffusion barrier maintains distribution of membrane proteins in polarized neurons. Nature. 1999;397:698–701. doi: 10.1038/17806. [DOI] [PubMed] [Google Scholar]
- 56.Xu M, Cao R, Xiao R, Zhu MX, Gu C. The axon-dendrite targeting of Kv3 (Shaw) channels is determined by a targeting motif that associates with the T1 domain and ankyrin G. J Neurosci. 2007;27:14158–14170. doi: 10.1523/JNEUROSCI.3675-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang CL, Messing A, Chiu SY. Specific alteration of spontaneous GABAergic inhibition in cerebellar purkinje cells in mice lacking the potassium channel Kv1. 1. J Neurosci. 1999;19:2852–2864. doi: 10.1523/JNEUROSCI.19-08-02852.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zonta B, Tait S, Melrose S, Anderson H, Harroch S, Higginson J, Sherman DL, Brophy PJ. Glial and neuronal isoforms of Neurofascin have distinct roles in the assembly of nodes of Ranvier in the central nervous system. J Cell Biol. 2008;181:1169–1177. doi: 10.1083/jcb.200712154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zweier C, de Jong EK, Zweier M, Orrico A, Ousager LB, Collins AL, Bijlsma EK, Oortveld MA, Ekici AB, Reis A, Schenck A, Rauch A. CNTNAP2 and NRXN1 are mutated in autosomal-recessive Pitt-Hopkins-like mental retardation and determine the level of a common synaptic protein in Drosophila. Am J Hum Genet. 2009;85:655–666. doi: 10.1016/j.ajhg.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]