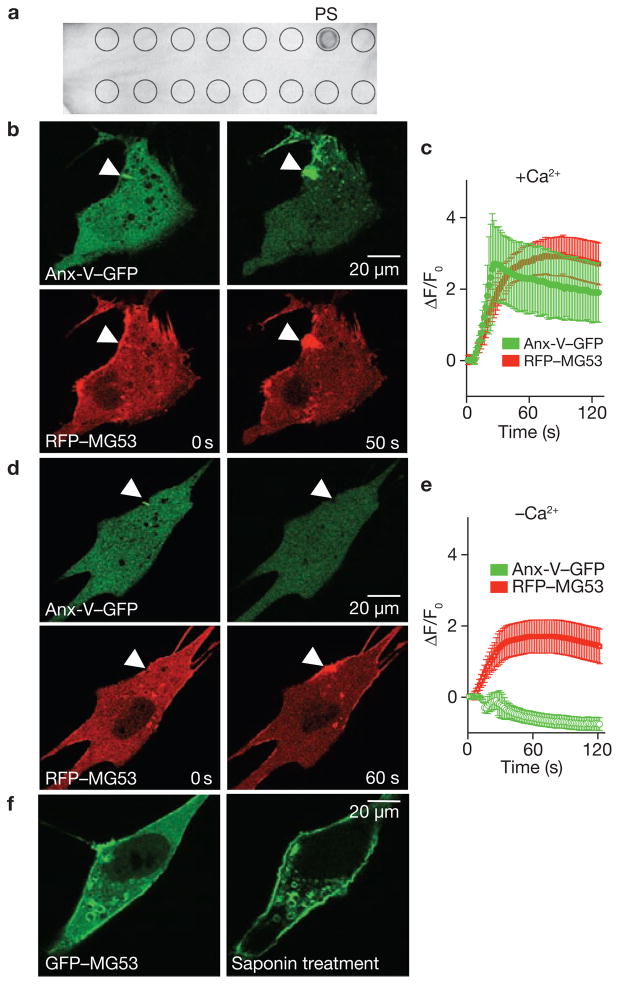
Figure 6.
MG53 binds to phosphatidylserine (PS) to mediate Ca2+-independent vesicle translocation to the injury site. (a ) PIP2-Strip lipid dot blot analysis reveals recombinant MG53 (1 μg ml−1) binds PS but not other membrane lipids, including sphingosine-1-P, phosphatidic acid, phosphotidylcholine, phosphatidylethanolamine and various phosphainositol metabolites. (b) Microelectrode penetration of C2C12 cells co-expressing annexin-V–GFP (top) and RFP–MG53 (bottom) in the presence of 2 mM Ca2+o results in colocalization of annexin-V and MG53 at the injury site. (c) Time-course of annexin-V–GFP and RFP–MG53 accumulation at injury sites following microelectrode penetration into C2C12 cells. RFP–MG53 continued to accumulate at the injury site, whereas annexin-V–GFP accumulation seemed to be biphasic. Membrane resealing probably reduces Ca2+ entry-dependent binding of annexin to PS-enriched membrane surfaces at later time points. Data represent mean ± s.e.m. (n = 16). (d) Removal of Ca2+o prevents movement of annexin-V–GFP (top) to the injury site (arrow), whereas RFP–MG53 can still translocate following membrane disruption (bottom). (e) Time-dependent changes for accumulation of RGP–MG53 and annexin-V–GFP following acute injury of C2C12 cells in the absence of Ca2+o plus 0.5 mM EGTA. Data represent mean ± s.e.m., n = 12. (f) C2C12 myoblasts transfected with GFP–MG53 show translocation of GFP–MG53 to the plasma membrane following treatment with 0.005% saponin in an extracellular solution containing 0 Ca2+ plus 0.5 mM EGTA.