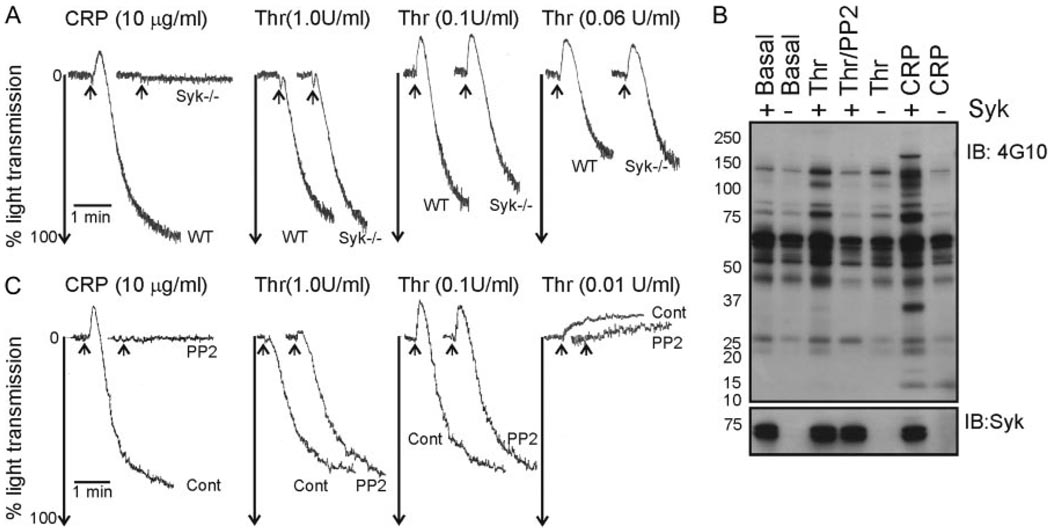
Figure 2.
Src and Syk kinases are required for optimal tyrosine phosphorylation in thrombin-stimulated platelets, but not aggregation. A and B, Platelets from wild-type (WT) or Syk-deficient (Syk −/−) mice were aggregated to thrombin (0.06 to 1.0U/mL, Thr) or CRP (10 µg/mL). The extent of aggregation was determined by change in light transmission. B, Washed platelets were treated with lotrafiban (10 µmol/L) then stirred in the absence (Basal), or presence of thrombin (1.0U/mL), thrombin/PP2 (20 µmol/L), or CRP (3 µg/mL). Stimulations were terminated and whole cell lysates were separated by SDS-PAGE, transferred and immunoblotted with antiphosphotyrosine (4G10) and anti-Syk antibodies. C, Washed human platelets were aggregated with thrombin (0.01 to 1.0U/mL, Cont), ± PP2 (10 µmol/L). Arrows mark the addition of agonist. These results are representative of at least 2 experiments using pooled murine platelets.