Abstract
Background and Aims
Reproductive costs imply trade-offs in resource distribution at the physiological level, expressed as changes in future growth and/or reproduction. In dioecious species, females generally endure higher reproductive effort, although this is not necessarily expressed through higher somatic costs, as compensatory mechanisms may foster resource uptake during reproduction.
Methods
To assess effects of reproductive allocation on vegetative growth and physiological response in terms of costs and compensation mechanisms, a manipulative experiment of inflorescence bud removal was carried out in the sexually dimorphic species Corema album. Over two consecutive growing seasons, vegetative growth patterns, water status and photochemical efficiency were measured to evaluate gender-related differences.
Key Results
Suppression of reproductive allocation resulted in a direct reduction in somatic costs of reproduction, expressed through changes in growth variables and plant physiological status. Inflorescence bud removal was related to an increase in shoot elongation and water potential in male and female plants. The response to inflorescence bud removal showed gender-related differences that were related to the moment of maximum reproductive effort in each sexual form: flowering in males and fruiting in females. Delayed costs of reproduction were found in both water status and growth variables, showing gender-related differences in resource storage and use.
Conclusions
Results are consistent with the existence of a trade-off between reproductive and vegetative biomass, indicating that reproduction and growth depend on the same resource pool. Gender-related morphological and physiological differences arise as a response to different reproductive resource requirements. Delayed somatic costs provide evidence of gender-related differences in resource allocation and storage. Adaptive differences between genders in C. album may arise through the development of mechanisms which compensate for the cost of reproduction.
Keywords: Corema album, costs of reproduction, flower removal, photochemical efficiency, sexual dimorphism, trade-off, water potential
INTRODUCTION
The reproductive costs hypothesis states that plant resource allocation or development is limited such that an increase in ongoing plant reproduction incurs a reduction in fitness, quantified as reduced future fecundity or survival (Levins, 1968; Karlsson and Méndez, 2005). The main assumption in the hypothesis of reproductive costs is that a trade-off occurs in the distribution of resources at the physiological level, either towards vegetative growth or towards reproduction. Thus, it could be expected that plants express physiological costs of reproduction through a reduction in growth or in future reproduction (Fox, 1995).
Dioecious plants, in which reproductive functions are separated in two distinct sexual forms, are especially suitable for studying interactions between growth and reproduction, as the effects of flower and fruit production can be analysed separately (Hoffmann and Alliende, 1984). In dioecious woody species, the timing and extent of the reproductive cost are reported to differ between sexual forms, with a general pattern of females enduring a higher net total cost than males (Leigh et al., 2006). Intersexual differences in reproductive allocation can result in relative differences in life-history traits, such as stem growth and flower and fruit production (Matsuyama and Sakimoto, 2008).
Nevertheless, reproduction may not always incur a detectable cost, because compensatory mechanisms may exist to foster resource uptake during reproduction (Tuomi et al., 1983), rendering these intersexual differences in reproductive trade-offs non-detectable. Several mechanisms which compensate for higher reproductive allocation in females have been described in dioecious species; these include (a) different timing of vegetative and reproductive allocation (Delph, 1990; Obeso, 2002; Milla et al., 2006), (b) microhabitat partitioning among the sexes (Bierzychudek and Eckhart, 1988; Dawson and Geber, 1999) and (c) increased photosynthetic activity during reproduction (Dawson and Ehleringer, 1993; Obeso, 2002; Nicotra et al., 2003).
In this context and assuming equal conditions, female individuals should have greater capacity than males for resource acquisition through uptake, assimilation and/or reabsorption throughout the reproductive period, but particularly during fruiting, when greater resource allocation occurs (Ashman, 1994; Delph, 1999).
Sequencing of vegetative growth and fruiting, or non-overlapping phenophases, has been described to be a compensating mechanism, reducing competition for resources between reproductive and vegetative organs (Delph, 1990; Matsuyama and Sakimoto, 2008). Differences in the timing of vegetative growth and reproduction have been described to vary between sexual forms in an array of woody dioecious species (Ågren, 1988; Popp and Reinartz, 1988; Matsuyama and Sakimoto, 2008). In Pistacea lentiscus, Milla et al. (2006) described the sequencing of flowering and growing periods to explain the lack of trade-offs between reproduction and vegetative growth, diminishing reproductive costs. In the dioecious shrub Corema album, Zunzunegui et al. (2006) found evidence of gender differences in the timing of maximum vegetative growth and reproductive investment within a growing season. Early growth in females has been explained by several authors as a compensation for their greater resource needs for fruit production, allowing a greater allocation of resources to storage organs and/or the production of a greater leaf area before the subsequent reproductive event (Popp and Reinartz, 1988; Delph, 1990; Obeso, 2002; Matsuyama and Sakimoto, 2008).
Manipulative experiments have been used to assess balances in resource allocation and reproductive costs, as they constitute a fixed environmental effect independent of the genetic origin, and therefore allows isolation of the indirect demographic costs of adaptations that compensate for reproductive costs (Hartemink et al., 2004; Andersson, 2005; Gehring and Delph, 2006; Narbona and Dirzo, 2010; among others). However, experiments employing direct manipulation of reproduction may be limited in terms of describing trade-offs, if storage organs such as stems or roots compete with reproductive structures as sinks altering the sink : resource ratio (allocation to reproductive vs. photosynthetic tissues). This has been described as being of particular importance in seasonal habitats (Ehrlén and van Groenendael, 2001). Thus, studies spanning at least two reproductive events are considered to be more accurate in detecting the effects of manipulations of current reproductive allocation on future growth and/or reproduction (Primack and Hall, 1990; Ehrlén and van Groenendael, 2001; García and Ehrlén, 2002; Horibata et al., 2007). In addition, very few studies have analysed the effects of reproductive allocation on growth and physiological response concomitantly (but see Dumka et al., 2003; Horibata et al., 2007).
In the present study, a direct manipulation experiment involving inflorescence bud removal was performed with the aim of analysing reproductive costs on vegetative growth and physiological response of male and female Corema album individuals, determining the effects of current reproductive allocation over two consecutive years. In particular, the following questions were addressed. (a) Are sexual forms different in terms of trade-offs between reproduction and vegetative growth? (b) Is there a physiological mechanism compensating for reproductive costs? (c) Does reproduction cause indirect (delayed) somatic or physiological costs? A greater effect of inflorescence bud removal on vegetative growth and physiology on female individuals was expected, as this is the sexual form with the greatest reproductive allocation in C. album (Guitián et al., 1997; Zunzunegui et al., 2006).
To test the existence of physiological compensatory mechanisms, leaf water potential and photochemical efficiency of chlorophyll a were determined, as it was anticipated that water relations and photosynthetic assimilation would be affected by the different reproductive demands between genders. Biomass allocation pattern was also measured, as this variable could be related to an increase in photosynthetic assimilation related to reproductive costs of both current and ensuing reproductive seasons (Case and Barrett, 2004; Milla et al., 2006).
MATERIALS AND METHODS
Study species
Corema album (Ericaceae) is an evergreen dioecious shrub which rarely exceeds 1 m in height. The species is also considered as subdioecious because male plants occasionally present inflorescences with staminate flowers only and inflorescences with both pistillate and staminate flowers, although hermaphrodite individuals are scarce (1–4 %) and only present at the southern most populations of the species’ geographical range (Zunzunegui et al., 2006). Leaves are ericoid, alternated or verticillated and persist for only two growing seasons. Pollination is anemophilous and fruits are spherical white or pink–white berries (5–8 mm in diameter). The growing period takes place from February to July, reaching its maximum between April and June, while flowering occurs from February to April, with fruits ripening from June to July. Corema album is endemic to the Atlantic coast of the Iberian Peninsula, from north-west Spain to the proximities of the Straits of Gibraltar in the south, growing on sandy soils over coastal dunes and cliffs (Valdés et al., 1987).
Previous studies have reported that reproductive effort is 3-fold higher in female than in male plants in terms of biomass at different populations along the geographical range of C. album: Guitián et al. (1997) in the north of the species range and Zunzunegui et al. (2006) in the south at the same study site selected for the present study.
Study site
The study was carried out in the coastal dunes of El Asperillo (34 °0’N 6 °36’W), in the Doñana Natural Park (south-west Spain). The climate is Mediterranean type with oceanic influence and mild temperatures. Mean annual temperature is 16·8 °C. Average annual rainfall is 550 mm, concentrated between October and March. Vegetation consists of native dune scrub of Halimium halimifolium, H. commutatum, Cistus libanotis, C. salvifolius, Rosmarinus officinalis, Lavandula stoechas, Cytisus grandiflorus, Stauracanthus genistoides, Corema album, Juniperus oxycedrus and J. phoenicea, together with Pinus pinea. Corema album is the dominant scrub in some areas (Díaz Barradas and Muñoz Reinoso, 1992).
The study was conducted from February to July over two consecutive growing periods, 2004 and 2005. The second study period was especially dry, with an annual rainfall 60 % below the historic yearly average for the area (179 mm).
Experimental design
Ten even-sized individuals of each gender were randomly chosen and marked within a 50 × 50 m area, where male and female canopies show a random spatial distribution and the sex ratio is equilibrated (Álvarez-Cansino, 2009). At the beginning of 2004, all inflorescence buds were removed, on initial appearance and before flowers could bloom (in January for male individuals and in February for females), with the aim of suppressing reproduction. Ten other similar individuals of each gender were marked for control purposes in the same area. All selected individuals were reproductive in the two study years and no hermaphrodite flowers were detected on any of them.
Gender determination was performed by searching for remains of anthers and fruits from the previous reproductive period. Residual late inflorescences were systematically removed over a 2-week period after the start of the study.
Measurements of major (A) and minor (a) axes (A orthogonal with respect to a) of canopy projection were carried out on each selected individual. Canopy area was calculated approaching canopy projection to an ellipse. The range of canopy size overlapped between sexes (range of mean values of A and a: 0·5–1·025 m for males and 0·49–1·03 m for females), with no significant differences between genders (ANOVA, F1 = 0·663, P = 0·426).
To estimate the number of flowers produced per plant, all inflorescence buds removed at the beginning of the experiment (2004) were counted and the number of flowers per square metre of canopy was calculated with the area projection of each individual.
To estimate the number of fruits produced per plant, and account for inter-annual variation in fruit production, two 0·25 m2 quadrats were randomly placed in the canopy of each control plant when fruits had ripened (July) in the 2004 and 2005 reproductive events. All fruits within the quadrats were counted and the average number of fruits per individual calculated.
Shoot elongation
In C. album, growth of new branches occurs after flowering, generating a bud-scale scar in the stem. In each of the 40 marked individuals, ten randomly chosen branches were marked in the last bud scar (10 branches per 10 individuals per 2 genders per 2 treatments; n = 400), and the growth of newly produced shoots was measured over two consecutive growing periods following Gibson and Menges (1994). Measurements were made fortnightly throughout the 2004, and monthly throughout the 2005 growing periods (February to July), with the aim of studying the effects of inflorescence bud removal during the current and subsequent growing periods. To calculate inflorescence bud removal effects on vegetative allocation, the following measurements were conducted on each branch.
Shoot elongation (SE) of newly produced shoots was calculated separately for each of the ten marked branches per plant as the sum of the elongation values of all the ramifications produced for each new shoot at each measuring date following Zunzunegui et al. (2002). The mean SE per individual was calculated for each sampling date (10 individuals × 2 genders × 2 treatments; n = 40).
Shoot elongation rate (SErate, mm d−1) was calculated as the increase in shoot length between two consecutive measuring dates divided by the number of days elapsed between them:
| (1) |
Accumulated shoot elongation at the end of the growing period (SEa, mm) was measured as the final SE value accumulated per shoot at the end of each study year (2004 and 2005).
The average number of shoots produced per measured branch was calculated for an estimate of ramification per individual.
Mean shoot elongation per shoot (MSE, mm) through the whole growing season was estimated as the ratio between SE and the number of ramifications per terminal shoot (eqn 2).
| (2) |
Biomass production
To determine biomass production per shoot, at the end of the experiment all the new shoots produced in the 2005 growing season from all branches marked in 2004 were cut at the level of the last bud-scale scar, which was produced during the 2005 flowering event (n = 400, 10 branches per 10 individuals per 2 gender per 2 treatment). After drying in a forced-air oven at 80 °C for 24 h, plant material was separated into leaves, stems and fruits (in 2005, buds were not removed, hence, fruits for both treatments were available) and weighed for the estimation of relative biomass devoted to fruit production as:
fruits dry weight (DW)/vegetative DW (leaves + stems) per shoot
Physiological measurements
The following physiological measurements were taken from each of the 40 marked individuals (10 individuals per 2 genders per 2 treatments) in February (beginning of flowering and coldest month), May (beginning of fruit ripening) and July (end of the growing season and fruit ripening and summer drought) in both study years.
Water potential was measured on one terminal twig per individual using a Scholander pressure chamber (Scholander et al., 1965).
Photochemical efficiency of chlorophyll a (photosystem II) was measured using a portable modulated fluorimeter (MINI-PAM, Walz Effeltrich, Germany) following the modulated pulse-amplitude technique (Bilger et al., 1995). Calculations followed Schreiber et al. (1995). Maximum photochemical efficiency of chlorophyll a (Fv/Fm) was measured on leaves previously adapted to darkness for 20 min (three twigs per 40 individuals). The average value of the three Fv/Fm measurements was calculated for each individual (n = 40).
Both water potential and photochemical efficiency of chlorophyll a were measured at solar midday on sunny days, the time of maximum environmental stress, with the purpose of recording the eventual physiological differences related to reproductive effort.
Data analysis
Differences in shoot elongation rate (SErate) between males and females and between controls and bud-removed plants were analysed by means of a repeated-measures ANOVA with gender and treatment as fixed factors. Due to the effect of plant size in determining the patterns of reproductive allocation (Bañuelos and Obeso, 2004; Friedman and Barrett, 2009) individual canopy size was included as a covariate. In addition, two-way ANOVAs were performed separately for each measurement month, with the aim of detecting the effect of inflorescence bud removal on the different phases of the reproductive season.
Although the branches of some species can be considered as modules or independent units (Tuomi et al., 1982), in order to consider the effect of individual plants and take into account possible inter-individual differences in size and/or microsite effects, a nested ANOVA was carried out to compare differences among treatments of mean shoot elongation (MSE), accumulated shoot elongation (SEa) and biomass production data. Treatment was considered as the fixed factor and individuals as the random factor nested in treatment. To compare differences between genders in each treatment, a nested ANOVA was carried out, with gender as the fixed factor and individual as random factor nested in gender.
The possible effect of differences in shoot size on the effect of the experimental treatment on MSE, SEa and biomass production variables was previously dismissed by means of a Levene's test for equality of variance. Homoscedasticity of variance was supported in shoot size for both study periods: 2004 (male, F = 0·631, P = 0·428; female, F = 1·866, P = 0·174) and 2005 (male, F = 0·232, P = 0·858; female, F = 1·352, P = 0·246).
Physiological measurements of water potential and photochemical efficiency of chlorophyll a were analysed by means of a repeated-measures ANOVA to contrast the interaction of sampling date on the effects of gender and treatment.
Normality of data was previously checked using a Kolmogorov–Smirnov non-parametric test. Shoot elongation rate, MSE and biomass production data were transformed by ln (n + 1) for a greater homogeneity in standard deviation and a better fit to the normal distribution. The non-normal variables number of ramifications and number of fruits were analysed for differences between treatments and genders by means of a Mann–Whitney's U non-parametric test.
All statistical analyses were performed using SPSS 17 (SPSS, Chicago, IL, USA) except nested ANOVA, for which the Statistica 6 (StatSoft, Tulsa, OK, USA) software package was used.
RESULTS
A total of 25 154 inflorescence buds was removed from 20 marked plants; 18 865 (75 %) from male plants and 6289 (25 %) from females. Male individuals exhibited an average of 3960 ± 492 inflorescences m−2 canopy, significantly greater than the values for female plants, with 1148 ± 185 inflorescences m−2 canopy (F1 = 31·23, P < 0·001). These data are consistent with previous results from the authors in studies of C. album (Zunzunegui et al., 2006).
Fruit production in control plants showed an inter-annual variation, with lower values in 2004 (1651 ± 375 fruits m−2 canopy) compared with 2005 (3855 ± 592 fruits m−2 canopy) (F = 8·73, P < 0·05).
Effect of inflorescence bud removal on shoot elongation
Bud removal had a significant effect on SErate over the two growing seasons studied. Repeated-measures ANOVA indicated the existence of a significant interaction between month, gender and treatment; thus, the intensity of monthly SErate depended both on the treatment and the gender (Table 1).
Table 1.
Results of repeated-measures ANOVA for the effect of treatment and gender on shoot elongation rate (SErate), leaf water potential and maximum photochemical efficiency (Fv/Fm)
| 2004 |
2005 |
||||||
|---|---|---|---|---|---|---|---|
| Variable | Comparison | MS | F | P | MS | F | P |
| Shoot elongation rate | Month | 0·520 | 5·963† | 0·001*** | 0·543 | 11·460‡ | 0·001*** |
| Month × gender | 0·539 | 6·43† | 0·001*** | 0·728 | 26·796‡ | 0·001*** | |
| Month × treatment | 0·311 | 2·486† | 0·043** | 0·245 | 3·243‡ | 0·021** | |
| Month × gender × treatment | 0·603 | 8·345† | 0·001*** | 0·222 | 2·846‡ | 0·036** | |
| Leaf water potential | Month | 1·25 | 23·51 | 0·001*** | 0·92 | 137·27 | 0·001*** |
| Month × gender | 0·006 | 0·11 | 0·890 | 0·482 | 11·18 | 0·001*** | |
| Month × treatment | 0·06 | 1·127 | 0·332 | 0·177 | 2·58 | 0·096* | |
| Month × gender × treatment | 0·035 | 0·652 | 0·525 | 0·122 | 1·67 | 0·209 | |
| Maximum photochemical efficiency | Month | 0·022 | 20·46 | 0·001*** | 0·91 | 126·8 | 0·001*** |
| Month × gender | 0·000 | 0·97 | 0·908 | 0·05 | 0·659 | 0·526 | |
| Month × treatment | 0·001 | 6·36 | 0·572 | 0·03 | 0·434 | 0·653 | |
| Month × gender × treatment | 0·001 | 5·46 | 0·349 | 0·07 | 0·966 | 0·394 | |
F, 2 degrees of freedom, except where indicated otherwise: † d.f. = 7 and ‡ d.f. = 5.
Asterisks indicate significant differences (* P < 0·1; ** P < 0·05; *** P < 0·01).
Bud-removed male individuals (Mbr) showed greater shoot elongation rate during the first flowering period (March to April 2004; Fig. 1); during the rest of the cycle, the elongation rate was greater in control males (Mc), although differences were not significant. Bud-removed female individuals (Fbr) showed greater elongation rates than control females (Fc) during fruit production (May to June), although differences were not significant. In July, once the fruits were formed, Fc showed higher values than Fbr (Fig. 1). In 2005, Fbr individuals showed greater SErate than controls from March to May, while in males the differences between treatments were not significant (Fig. 1).
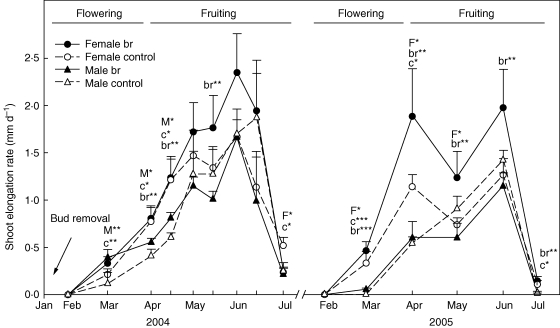
Fig. 1.
Shoot elongation rate (SErate, mm d−1) in male and female individuals [control and bud-removed (br)] throughout the 2004 and 2005 growing periods (mean + s.e.). Upper-case letters indicate significant differences between treatments (M for male plants and F for female plants); lower-case letters indicate significant differences between sexes (c for control treatment and br for bud-removed). Asterisks indicate significant differences (* P < 0·05; ** P < 0·01, *** P < 0·001). The length of flowering and fruiting periods is shown.
Between genders, two-way ANOVA revealed significant differences in both bud-removed and control individuals in both growing seasons. Fbr individuals showed growth rates equal to or higher than Mbr, which were more evident throughout the second growing period after bud removal (Fig. 1). In control individuals, Fc showed higher shoot elongation rates than Mc at the beginning of the two growing seasons. At the end of the growing season (July), after fruit formation period, Fc individuals again showed higher elongation rates than Mc in both study years (Fig. 1).
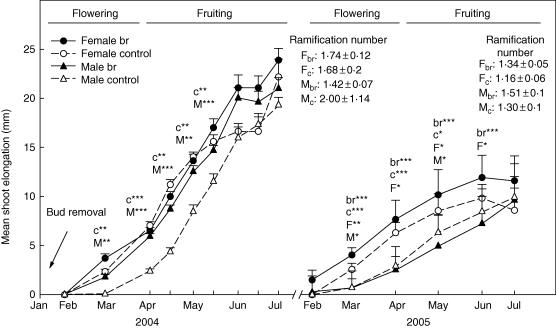
One-way nested ANOVA showed significant differences between treatments in MSE in both genders (Fig. 2). Control male individuals had lower MSE than bud-removed males during most of the 2004 growing season while, in 2005, control males had a greater MSE in March and May. In female individuals, bud-removed plants had greater MSE than controls only in 2005. Between genders, in the 2004 growing season (March to May), the nested ANOVA showed lower MSE in male control individuals compared with female controls. No significant differences between genders were found in MSE in bud-removed individuals. In 2005, significant differences between genders were found in both treatments, with greater MSE values in female individuals.
Fig. 2.
Mean shoot elongation per shoot (MSE) + standard error in both treatments for male and female individuals throughout the 2004 and 2005 growing periods. Mean ramifications number ± s.d. is also shown for each treatment and gender. Upper-case letters indicate significant differences between treatments (M for males plants and F for female plants); lower-case letters indicate significant differences between sexes (c for control treatment and br for bud-removed). Asterisks indicate significant differences (* P < 0·05; **P<0·01, *** P < 0·001). The length of flowering and fruiting periods is shown.
Similar to the MSE results, Mann–Whitney's U non-parametric test showed a significantly greater number of ramifications per terminal shoot for control males in comparison with bud-removed males at the end of the growing season, though only in the first year. No differences were found between treatments in female individuals (Fig. 2 and Table 2). Between genders, control males tended to show a higher number of ramifications than females in 2004, although differences were not significant. Significant differences were found in both treatments in 2005, with male individuals showing a greater number of ramifications than females.
Table 2.
Results of the non-parametric Mann–Whitney's U test for the effects of treatment and gender on number of ramifications in 2004 and 2005
| 2004 |
2005 |
||||
|---|---|---|---|---|---|
| Source of variation | Comparison | U | P | U | P |
| Treatment | Male | 2·00 | 0·005** | 29·50 | 0·205 |
| Female | 19·50 | 0·498 | 39·00 | 0·258 | |
| Gender | Bud-removed | 33·00 | 0·196 | 17·00 | 0·022* |
| Control | 12·00 | 0·916 | 21·50 | 0·018* | |
Asterisks indicate significant differences (* P < 0·05;** P < 0·01).
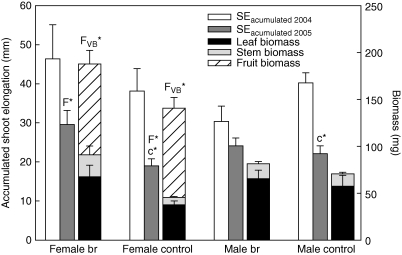
Accumulated shoot elongation at the end of the growing period (SEa) was greater in bud-removed female individuals in both study years, although one-way nested ANOVA only showed significant differences between treatments in 2005 (Fig. 3 and Table 3). In male individuals, no differences between treatments were found in any of the study years.
Fig. 3.
Accumulated shoot elongation (SEa), biomass production (stem, leaves and fruits dry weight) per branch at the end of the growing season. Mean + s.e. Upper-case letter F indicates significant differences between treatments for female plants; FVB indicate differences in vegetative biomass in female plants; lower-case letter c indicates significant differences between genders for control treatment. Asterisks indicate significant differences (* P < 0·05).
Table 3.
Results of one-way nested ANOVA for the effect of treatment and gender on SEa
| 2004 |
2005 |
||||||
|---|---|---|---|---|---|---|---|
| Source of variation | Comparison | MS | F | P | MS | F | P |
| Treatment | Male | 1·134 | 2·878 | 0·114 | 0·013 | 0·026 | 0·873 |
| Female | 0·241 | 0·200 | 0·662 | 5·043 | 7·752 | 0·012* | |
| Gender | Bud-removed | 1·810 | 1·679 | 0·211 | 0·015 | 0·019 | 0·892 |
| Control | 0·267 | 0·916 | 0·507 | 1·878 | 6·268 | 0·023* | |
F, 1 degree of freedom.
Asterisks indicate significant differences (* P < 0·05).
Bud-removed female individuals tended to have a higher SEa than males in both study years, but differences were not significant (Fig. 3). In control individuals, males tended to have a greater SEa in both years, with significant differences also present in 2005.
SEa was lower in both genders and treatments in 2005 compared with 2004, due to the severe drought which occurred during this second period.
Effect of inflorescence bud removal on biomass production
Inflorescence bud removal showed a significant effect on vegetative DW (leaves plus stems), but only in female individuals (Fig. 3). No significant differences were found between genders on biomass production.
In females, no significant differences between treatments were found at the end of the growing season in 2005, in fruit DW (Fig. 3), in the proportion of biomass DW devoted to fruits (Fbr, 1·35 ± 0·18, Fc, 1·82 ± 0·23), nor in the number of fruits (Mann–Whitney's U = 4365·00; P > 0·05).
Within single female individuals, no differences in biomass production were found comparing fruit-bearing with fruitless branches in any of the treatments (P > 0·05; data not shown).
Physiological response to inflorescence bud removal
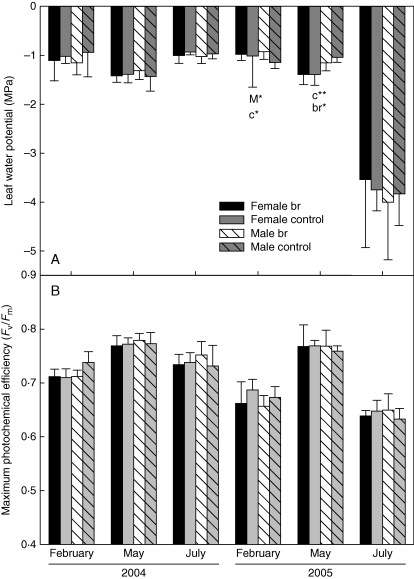
Repeated-measures ANOVA showed the existence of significant month–gender and month–treatment interactions in 2005 (P < 0·1), indicating that monthly variation in leaf water potential was gender dependent and was also affected by suppression of reproduction (Table 1). Accordingly, one-way ANOVA showed that inflorescence bud removal had a significant effect on water potential in 2005; in February, control male individuals showed lower values than bud-removed males (P < 0·05; Fig. 4A). Between the genders, male individuals showed significantly lower values than females in February in the control treatment (P < 0·05; Fig. 4A); conversely, in May, female individuals showed lower values than males for both treatments (P < 0·01 and P < 0·05, respectively; Fig. 4A).
Fig. 4.
Leaf water potential (A) and maximum photochemical efficiency (B) at the three sampling dates in 2004 and 2005. Mean values ± s.e. are shown. Upper-case letter M with asterisks indicates significant differences between treatments for male plants, lower-case letters indicate significant differences between genders (c, control; br, bud-removed plants). Asterisks indicate significant differences (* P < 0·05; ** P < 0·01).
Repeated-measures ANOVA on the variable maximum photochemical efficiency (Fv/Fm) indicated significant differences between months only, with no interactions between genders or treatments (Fig. 4B and Table 1). In 2004, both genders showed Fv/Fm values around 0·7 at all sampling dates, very close to the optimum, set between 0·7 and 0·8 by several authors (Björkman and Demmig, 1987; Valladares et al., 2000). In February and July of 2005, Fv/Fm values under the physiological optimum were recorded.
DISCUSSION
Trade-offs between reproduction and vegetative growth
Suppression of reproductive allocation resulted in an increased shoot elongation rate during the reproductive period in both genders, indicating a direct reduction in the somatic cost of reproduction. According to the results, bud-removed female (Fbr) individuals showed higher growth rates than bud-removed males (Mbr) in May, when fruits start to form, and during the whole period in the following growing season (Fig. 1). The results demonstrate that the effect of inflorescence bud removal was higher in the gender bearing the greatest reproductive effort.
Throughout the reproductive event, gender differences found in the growth rate response to inflorescence bud removal can be explained through the differences in flowering and fruiting periods in each sexual form. Gender-related variations in growth investment that reflect reproduction timing are common in dioecious species (Obeso, 2002, and references therein), in which the vegetative growth of female individuals surpasses that of males during flowering, while shoot elongation of males surpasses that found in the females during fruiting (Delph, 1990).
The allocation of resources towards reproduction in control individuals involved a somatic cost that became apparent through a reduction in shoot elongation rate, following the same trend in the two study periods (Fig. 1 and Table 1). This finding is consistent with results obtained by the authors in previous years (Zunzunegui et al., 2006). Female individuals started to grow earlier than males and their growth rate increased gradually until the middle of May, when it decreased, coinciding with fruit production (Fig. 1). By the end of the growing season, both sexual forms had accumulated equal shoot elongations (Fig. 3). However, during the flowering period of control males (Mc) in 2004 and 2005 and of Mbr in 2005, their growth rate was lower than that of female plants (Fig. 1).
These results are consistent with the presence of a trade-off between investments in reproductive biomass and vegetative biomass, as has been described for other dioecious species (Vasiliauskas and Aarseen, 1992; Gibson and Menges, 1994). The negative effect of reproduction on growth, represented as reductions in elongation and accumulated shoot elongation (SEa) rates, indicates that both processes are at least partially dependent on the same resource pool (Nicotra, 1999).
In the present study, inflorescence bud removal is shown to have consequences on the current growing season but also delayed effects on the subsequent growing period, at the end of which the bud-removed females showed greater SEa than the controls (Fig. 3 and Table 3). Delayed effects of reproduction on growth have been studied in several species such as Siparuna longiflora, in which manipulation of flower and fruit production produced an increase in growth of female individuals that was noticeable in the subsequent growing period (Nicotra, 1999). In Aesculus californica, Newell (1991) described an indirect expenditure in fruit-bearing branches that had a delayed effect on reproduction and vegetative growth. In other species, such as Lindera benzoin or Salix alaxensis, manipulation under natural conditions revealed the existence of balances between current and subsequent reproductive events, although not with subsequent vegetative growth (Fox and Stevens, 1991; Cipollini and Whigham, 1994).
The fact that only female individuals showed evidence of resource allocation towards growth, when the reproductive investment of the previous period had been suppressed, could be explained by the existence of gender-specific differences in compensatory mechanisms of reproduction, promoted by the greater reproductive effort of the females. Previous studies have shown evidence of a longer root system in female C. album individuals; in the same study site, Álvarez-Cansino et al. (2010), using xylem water isotopic composition analysis, found evidence of deeper soil layer water extraction by females. Roots act as a resource storage system – mainly for carbohydrates – that can be mobilized and allocated to reproduction during the reproductive period (Ehrlén and van Groenendael, 2001); a more developed root system would thus allow a greater resource storage capacity in female individuals. Hence, we conclude that inflorescence bud removal would imply resource allocation to non-reproductive organs in the subsequent cycle, greater in females compared with male individuals. These results point out the importance of considering below-ground storage organs and water relations to assess compensating mechanisms of reproductive effort in further research.
The fact that females produced more fruits in 2005 may have reinforced the effect of the inflorescence bud removal on vegetative allocation. Control females would have less accumulated resources than Fbr and, under higher fruit production, the delayed effect on vegetative costs would be enhanced. This is in accordance with the pattern found by Milla et al. (2006), who measured higher biomass and nutrients accumulated in the branches of males of Pistacia lentiscus in the current-year shoots, resulting in a relatively higher level of resources for a stronger flowering effort in males during the subsequent reproduction period.
Inter-annual differences in fruit production have been described in many species (Hoffmann and Alliende, 1984), and the results concur with previous data described for C. album (Font Quer, 1992; Zunzunegui et al., 2006). In addition, 2005 was a drier than average year, which would also increase gender dimorphism in physiological responses, which have been described as being fostered under harsher environmental conditions (Obeso, 2002).
Inflorescence bud removal only had an effect on ramification in male individuals (Fig. 2 and Table 2), which suggests that gender dimorphism in some morphological traits, in addition to being genetically conditioned, could also be affected by reproductive status. The effect of inflorescence bud removal on ramification and on mean shoot elongation (MSE) could be explained by the fact that floral meristem production can affect the development of secondary vegetative meristems through hormone production (Lehtila, 2000), that could be present in different concentrations depending on the sex.
In accordance with the present results in C. album, several authors have explained the greater ramification of male individuals of anemophyllous species as an adaptation for improved pollen production and exposition (Friedman and Barrett, 2009). Male individuals of Simmodsia chinenesis (Kohorn, 1994) and of Leucadendron species (Bond and Midgley, 1988) exhibited shorter internodes as a consequence of a greater number of inflorescences relative to the females. We consistently found that male C. album individuals, which have greater numbers of flowers than the females, had more ramified branches with shorter internodes (Fig. 2).
Differences found between treatments in the biomass production of female individuals (Fig. 3) confirm the results of shoot elongation and support the existence of a balance in resource investment between reproduction and maintenance of vegetative growth which, in the case of female individuals, would have an effect lasting from the current growing period to the subsequent period (Ågren, 1988; Nicotra, 1999). Inflorescence bud removal implied an increase in vegetative biomass production, compared with control individuals in the subsequent growing period (2005), which only took place in females (Fig. 3). However, reproduction in the subsequent period was not affected by the suppression of flower buds, as the same number and dry weight of fruits were found for both treatments in this study (Fig. 3).
Physiological responses to inflorescence bud removal
Reductions in delayed reproductive costs can be related both to biomass allocation patterns and to compensation coupled to an increase in photosynthetic assimilation (Case and Barrett, 2004); therefore, we would expect a response of the photosynthetic system to the inflorescence bud removal performed in C. album. Moreover, individuals of the gender and treatment that invest most in reproduction would be expected to show greater physiological stress (Verdú et al., 2004).
According to the present results, inflorescence bud removal affected the water status of male individuals during flowering (February) in the year following that of flower removal (2005), with control individuals showing more negative water potential values than bud-removed (Fig. 4A and Table 1). Equally, differences between genders during this period were only found in control individuals. These differences could be related to the high photoinhibition found in males in a previous study (Álvarez-Cansino, 2010), which confirms that environmental conditions of low temperatures and high radiation cause a greater physiological stress in the gender that invests the greatest effort during flowering. Gender-related differences found in May 2005, when the females showed lower leaf water potential values than the males, were more marked between control plants than between the bud-removed individuals. However, these differences were not correlated with differences in the photosynthetic response, measured as maximum photochemical efficiency of chlorophyll a (Fv/Fm) (Fig. 4B and Table 1). We could have expected an increase in photosynthesis in control plants relative to bud-removed individuals, which would contribute to the carbon allocated to the production of reproductive structures (Dawson and Ehleringer, 1993; Laporte and Delph, 1996). However, the large reproductive investment may not have an effect on the photosynthetic rate if plants respond by increasing their resource uptake or by using resources more efficiently. Many studies on sexually dimorphic species show no evidence of a local increase in photosynthesis due to reproduction, in which the reproductive expenditure could be compensated by the reallocation of resources from adjacent branches (Obeso et al., 1998). The complementary costs of increasing resource uptake could correspond to the costs of production and maintenance of roots or leaves (Case and Barrett, 2004).
In conclusion, the initial hypothesis that the effect of inflorescence bud removal would be greater in female individuals of C. album, which bear the greater reproductive effort due to fruit production, is confirmed. The results show evidence for differences between sexual forms in somatic reproductive costs and in the presence of delayed somatic costs, indicating gender-related differences in resource allocation and storage. In addition, it has been shown for the first time that gender-related morphological and physiological differences develop in C. album as a response to different reproductive resource requirements, and are thus dependent on reproductive status.
Long-term manipulative studies are confirmed as a useful method to assess reproductive costs and to explore the processes underlying the evolution of gender dimorphism in plants. This study gives new evidence of the importance of considering roots as essential storage and water control organs that may play a key role in reproductive allocation patterns and in the magnitude of gender dimorphism in sexually dimorphic species.
ACKNOWLEDGEMENTS
We thank the Junta de Andalucía for granting access permits to the Doñana Natural Park and to J. Jauregui for helpful comments on the manuscript. This work was supported by the Ministry of Education and Science (F.P.U. AP20021733 to L.A.-C.).
LITERATURE CITED
- Ågren J. Sexual differences in biomass and nutrient allocation in the dioecious Rubus chamaemorus. Ecology. 1988;69:962–973. [Google Scholar]
- Álvarez-Cansino L. PhD Thesis, University of Seville: Spain; 2009. Gender dimorphism in the dioecious shrub Corema album at population and biogeographical scales. [Google Scholar]
- Álvarez-Cansino L, Zunzunegui M, Díaz Barradas MC, Paz Esquivias M. Physiological performance and xylem water isotopic composition underlie gender-specific responses in the dioecious shrub Corema album. Physiologia Plantarum. 2010;140:32–45. doi: 10.1111/j.1399-3054.2010.01382.x. [DOI] [PubMed] [Google Scholar]
- Andersson S. Floral costs in Nigella sativa (Ranunculaceae): compensatory responses to perianth removal. American Journal of Botany. 2005;92:279–283. doi: 10.3732/ajb.92.2.279. [DOI] [PubMed] [Google Scholar]
- Ashman TL. A dynamic perspective on the physiological cost of reproduction in plants. American Naturalist. 1994;144:300–316. [Google Scholar]
- Bañuelos MJ, Obeso JR. Resource allocation in the dioecious shrub Rhamnus alpinus: the hidden costs of reproduction. Evolutionary Ecology Research. 2004;6:1–17. [Google Scholar]
- Bilger W, Schreiber U, Bock M. Determination of the quantum efficiency of photosystem II and of non-photochemical quenching of chlorophyll fluorescence in the field. Oecologia. 1995;102:425–432. doi: 10.1007/BF00341354. [DOI] [PubMed] [Google Scholar]
- Bierzychudek P, Eckhart V. Spatial segregation of the sexes of dioecious plants. American Naturalist. 1988;132:34–43. [Google Scholar]
- Björkman O, Demmig B. Photon yield of O2, evolution and chlorophyll fluorescence characteristics at 77K among vascular plants of diverse origins. Planta. 1987;170:489–504. doi: 10.1007/BF00402983. [DOI] [PubMed] [Google Scholar]
- Bond WJ, Midgley J. Allometry and sexual differences in leaf size. American Naturalist. 1988;131:901–910. [Google Scholar]
- Case AL, Barrett SCH. Environmental stress and the evolution of dioecy: Wurmbea dioica (Colchicaceae) in Western Australia. Evolutionary Ecology. 2004;18:145–164. [Google Scholar]
- Cipollini ML, Whigham DF. Sexual dimorphism and cost of reproduction in the dioecious shrub Lindera benzoin (Lauraceae) American Journal of Botany. 1994;81:65–75. [Google Scholar]
- Dawson TE, Ehleringer JR. Gender-specific physiology, carbon isotope discrimination, and habitat distribution in boxelder. Acer negundo. Ecology. 1993;74:798–815. [Google Scholar]
- Dawson TE, Geber MA. Sexual dimorphism in physiology and morphology. In: Geber MA, Dawson TE, Delph LF, editors. Gender and sexual dimorphism in flowering plants. Heidelberg: Springer-Verlag; 1999. pp. 175–215. [Google Scholar]
- Delph LF. Sex-differential resource allocation patterns in the subdioecious shrub Hebe subalpina. Ecology. 1990;71:1342–1351. [Google Scholar]
- Delph LF. Sexual dimorphism in life history. In: Geber MA, Dawson TE, Delph LF, editors. Gender and sexual dimorphism in flowering plants. Heidelberg: Springer-Verlag; 1999. pp. 149–173. [Google Scholar]
- Díaz Barradas MC, Muñoz Reinoso J. The ecology of vegetation of the Asperillo dune system, southwest Spain. In: Carter RWG, Curtis TGF, Sheehy-Skeffington MJ, editors. Coastal dunes: geomorphology, ecology and management. Rotterdam: Balkema; 1992. pp. 211–218. [Google Scholar]
- Dumka D, Bednarz CW, van Iersel MW. Effect of flower bud removal on carbon dioxide exchange rates of cotton. Communications in Soil Science and Plant Analysis. 2003;34:1611–1621. [Google Scholar]
- Ehrlén J, van Groenendael J. Storage and the delayed costs of reproduction in the understorey perennial Lathyrus vernus. Journal of Ecology. 2001;89:237–246. [Google Scholar]
- Font Quer P. Plantas medicinales. El Dioscórides renovado. Barcelona: Labor; 1992. [Google Scholar]
- Fox JF. Shoot demographic responses to manipulation of reproductive effort by bud removal in a willow. Oikos. 1995;72:283–287. [Google Scholar]
- Fox JF, Stevens GC. Costs of reproduction in a willow: experimental responses vs. natural variation. Ecology. 1991;72:1013–1023. [Google Scholar]
- Friedman J, Barrett SCH. Wind of change: new insights on the ecology and evolution of pollination and mating in wind-pollinated plants. Annals of Botany. 2009;103:1515–1527. doi: 10.1093/aob/mcp035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García MB, Ehrlén J. Reproductive effort and herbivory timing in a perennial herb: fitness components at the individual and population levels. American Journal of Botany. 2002;89:1295–1302. doi: 10.3732/ajb.89.8.1295. [DOI] [PubMed] [Google Scholar]
- Gehring JL, Delph LF. Effects of reduced source-sink ratio on the cost of reproduction in females of Silene latifolia. International Journal of Plant Sciences. 2006;167:843–851. [Google Scholar]
- Gibson DJ, Menges ES. Population structure and spatial pattern in the dioecious shrub Ceratiola ericoides. Journal of Vegetation Science. 1994;5:337–346. [Google Scholar]
- Guitián P, Medrano M, Rodríguez M. Reproductive biology of Corema album (L.) D. Don (Empetraceae) in the northwest Iberian Peninsula. Acta Botanica Gallica. 1997;144:119–128. [Google Scholar]
- Hartemink N, Jongejans E, de Kroon H. Flexible life history responses to flower and rosette bud removal in three perennial herbs. Oikos. 2004;105:159–167. [Google Scholar]
- Hoffmann AJ, Alliende MC. Interactions in the patterns of vegetative growth and reproduction in woody dioecious plants. Oecologia. 1984;61:109–114. doi: 10.1007/BF00379095. [DOI] [PubMed] [Google Scholar]
- Horibata S, Hasegawa SF, Kudo G. Cost of reproduction in a spring ephemeral species, Adonis ramosa (Ranunculaceae): carbon budget for seed production. Annals of Botany. 2007;100:565–571. doi: 10.1093/aob/mcm131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson P, Méndez M. The resource economy of plant reproduction. In: Reekie EG, Bazzaz FA, editors. Reproductive allocation in plants. London: Elsevier; 2005. pp. 1–49. [Google Scholar]
- Kohorn LU. Shoot morphology and reproduction in jojoba: advantages of sexual dimorphism. Ecology. 1994;75:2384–2394. [Google Scholar]
- Laporte MM, Delph LF. Sex specific physiology and source-sink relations in the dioecious plant Silene latifolia. Oecologia. 1996;106:63–72. doi: 10.1007/BF00334408. [DOI] [PubMed] [Google Scholar]
- Lehtila K. Modelling compensatory regrowth with bud dormancy and gradual activation of buds. Evolutionary Ecology. 2000;14:315–330. [Google Scholar]
- Leigh A, Cosgrove MJ, Nicotra AB. Reproductive allocation in a gender dimorphic shrub: anomalous female investment in Gynatrix pulchella? Journal of Ecology. 2006;94:1261–1271. [Google Scholar]
- Levins R. Evolution in changing environments. Princeton, NJ: Princeton University Press; 1968. [Google Scholar]
- Matsuyama S, Sakimoto M. Allocation to reproduction and relative reproductive costs in two species of dioecious Anacardiaceae with contrasting phenology. Annals of Botany. 2008;101:1391–1400. doi: 10.1093/aob/mcn048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milla R, Castro-Díez P, Maestro-Martínez M, Montserrat-Martí G. Costs of reproduction as related to the timing of phenological phases in the dioecious shrub Pistacea lentiscus L. Plant Biology. 2006;8:103–111. doi: 10.1055/s-2005-872890. [DOI] [PubMed] [Google Scholar]
- Narbona E, Dirzo R. Experimental defoliation affects male but not female reproductive performance of the tropical monoecious plant Croton suberosus (Euphorbiaceae) Annals of Botany. 2010;106:359–369. doi: 10.1093/aob/mcq117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell EA. Direct and delayed costs of reproduction in Aesculus californica. Journal of Ecology. 1991;79:365–378. [Google Scholar]
- Nicotra AB. Sexually dimorphic growth in the dioecious tropical shrub. Siparuna grandiflora. Functional Ecology. 1999;13:322–331. [Google Scholar]
- Nicotra AB, Chazdon RL, Montgomery RA. Sexes show contrasting patterns of leaf and crown carbon gain in a dioecious rainforest shrub. American Journal of Botany. 2003;90:347–355. doi: 10.3732/ajb.90.3.347. [DOI] [PubMed] [Google Scholar]
- Obeso JR. The costs of reproduction in plants. New Phytologist. 2002;155:321–348. doi: 10.1046/j.1469-8137.2002.00477.x. [DOI] [PubMed] [Google Scholar]
- Obeso JR, Álvarez-Santullano M, Retuerto R. Sex ratios, size distributions, and sexual dimorphism in the dioecious tree Ilex aquifolium (Aquifoliaceae) American Journal of Botany. 1998;85:1602–1608. [PubMed] [Google Scholar]
- Popp JW, Reinartz JA. Sexual dimorphism in biomass allocation and clonal growth of Xanthoxylum americanum. American Journal of Botany. 1988;75:1732–1741. [Google Scholar]
- Primack RB, Hall P. Costs of reproduction in the pink lady's slipper orchid: a four-year experimental study. American Naturalist. 1990;136:638–656. [Google Scholar]
- Scholander PF, Hammer HT, Bradstreet ED, Hemmingsen EA. Sap pressure in vascular plants. Science. 1965;148:339–346. doi: 10.1126/science.148.3668.339. [DOI] [PubMed] [Google Scholar]
- Schreiber U, Hormann H, Neubauer C, Klughammner C. Assesment of photosystem II photochemical quantum yield by chlorophyll fluorescence quenching analysis. Australian Journal of Plant Physiology. 1995;22:209–220. [Google Scholar]
- Tuomi J, Niemela P, Mannila R. Resource-allocation on dwarf shoots of birch (Betula pendula): reproduction and leaf growth. New Phytologist. 1982;91:483–487. [Google Scholar]
- Tuomi J, Hakala T, Haukioja E. Alternative concepts of reproductive effort, costs of reproduction, and selection in life-history evolution. American Zoologist. 1983;23:25–34. [Google Scholar]
- Valdés B, Talavera S, Fernández Galiano E. Flora vascular de Andalucía Occidental. Barcelona: Ketres; 1987. [Google Scholar]
- Valladares F, Wright SJ, Lasso E, Kitajima K, Pearcy RW. Plastic phenotypic response to light of 16 congeneric shurbs from a Panamanian rainforest. Ecology. 2000;81:1925–1936. [Google Scholar]
- Vasiliauskas SA, Aarseen LW. Sex ratio and neighbour effects in monospecific stands of Juniperus virginiana. Ecology. 1992;73:622–632. [Google Scholar]
- Verdú M, Villar-Salvador P, García-Fayos P. Gender effects on the post-facilitation performance of two dioecious Juniperus species. Functional Ecology. 2004;18:87–93. [Google Scholar]
- Zunzunegui M, Díaz Barradas MC, Aguilar Silva FJ, Ain Lhout F, Clavijo A, García Novo F. Growth response of Halimium halimifolium at four sites with different soil water availability regimes in two contrasted hydrological cycles. Plant and Soil. 2002;247:271–281. [Google Scholar]
- Zunzunegui M, Díaz Barradas MC, Clavijo A, Álvarez-Cansino L, Ain Lhout F, García Novo F. Ecophysiology, growth timing and reproductive effort of three sexual forms of Corema album. Plant Ecology. 2006;183:35–46. [Google Scholar]