Abstract
Purpose
Waldenstrom's macroglobulinemia (WM) is a lymphoplasmacytic lymphoma characterized by widespread involvement of the bone marrow. Despite different options of therapy, WM is still incurable. Src tyrosine kinase was shown to play a central role in the regulation of a variety of biological processes such as cell proliferation, migration, adhesion, and survival, in solid tumors. We sought to determine whether the protein tyrosine kinase Src regulates adhesion, migration and survival in WM.
Experimental Design
We have tested the expression of Src tyrosine kinase in WM and normal cells, and tested the effect of its specific inhibitor AZD-530 on adhesion, migration, cell cycle and survival of WM cell line and patient samples. Moreover, we tested its effect on sytockeletal and cell cycle signaling in WM.
Results
We demonstrated that Src is over expressed in WM cells compared to control B cells. And that the use of the Src inhibitor AZD0530 led to significant inhibition of adhesion, migration and cytosekletal signaling induced by SDF1. Moreover, inhibition of Src activity induced G1 cell cycle arrest; however, it had minimal effect on survival of WM cells, and no significant effect on survival of normal cells.
Conclusions
Taken together, these studies delineate the role of Src kinase activity in WM and provide the framework for future clinical trials using Src inhibitors in combination with other drugs to improve the outcome of patients with WM.
Keywords: Waldenstrom Macroglobulinemia, Src, adhesion, migration, homing, survival
INTRODUCTION
Src was the first oncogene to be discovered, as well as the first protein tyrosine kinase (1-5). It is a non-receptor tyrosine kinase that belongs to a nine-member family of kinases including Src, Yes, Fyn, Lyn, Lck, Hck, Fgr, Blk, and Yrk (6, 7). Src has been implicated in cell proliferation, survival, adhesion, migration, invasion, inflammation, and angiogenesis. Src is activated by growth factor receptors, cytokine receptors, and focal adhesion kinase (FAK) (8). Src interacts with a network of intracellular signaling pathways, including the integrin/FAK pathway, β-catenin/Wnt, RAS-MEK, PI3K–AKT and Janus-activated kinase–STAT pathways (7).
c-Src is over expressed and activated in a large number of human malignancies; it has been linked to the development of cancer and progression to distant metastases, specifically in gastrointestinal malignancies (5). However, the precise functions of c-Src in cancer remain unclear. In addition to increasing cell proliferation, key roles c-Src has in cancer seems to be regulating adhesion, invasion and motility, particularly in cancer cells during the later stages of cancer progression (9, 10). Ablation of Src family kinases inhibits tumor growth, as well as invasiveness and motility of cancer cells (11, 12). This observation prompted the advancement in a number of small-molecule Src kinase inhibitors that reduced cancer invasion and metastasis in preclinical models leading to the development of clinical trials using these agents. AZD0530, a potent and selective small-molecule inhibitor of Src kinase, is currently being tested in phase II clinical trials in patients with different malignancies (13, 14).
Waldenstrom Macroglobulinemia (WM) is a low-grade B cell malignancy characterized by the presence of lymphoplasmacytic cells in the bone marrow (BM) (15, 16). The specific tropism of these cells to the BM niches indicates their dependence on adhesion and critical interaction with the BM microenvironment(17). We previously demonstrated that the CXCR4/stromal derived factor (SDF-1) axis induces migration and adhesion in WM, and that SDF-1 is highly expressed in the bone marrow of WM patients (17).
In this study, we characterized the role of Src tyrosine kinase in migration, adhesion, cytoskeletal signaling, cell cycle and survival of WM cells. We further examined the role of Src in SDF1-induced signaling and functions in WM.
MATERIALS AND METHODS
Reagents
The Src inhibitor AZD0530 (AZD) was provided by AstraZeneca (Macclesfield, UK). AZD0530 was diluted in DMSO and stored at −20°C, and diluted in culture medium immediately before use. SDF-1 was purchased from R&D systems (Minneapolis, MN).
Cells
The WM cell line, BCWM1, is a kind gift from Dr. Steve Treon, Dana Farber Cancer Institute, Boston, MA, and has been developed from a patient with untreated IgM kappa WM(18). Cells were cultured in RPMI-1640 containing 10% fetal bovine serum (FBS, Sigma Chemical, St Louis, MO), 2mM L-glutamine, 100 U/mL penicillin, and 100μg/mL streptomycin (GIBCO, Grand Island, NY). The umbilical vein endothelial cells (HUVEC) (Cambrex, Walkersville, MD) were cultured in EGM-2 MV media (Cambrex) reconstituted according to the manufacturer instructions.
Patient samples were obtained after approval from the Dana-Farber Cancer Institute Institutional Review Board. Informed consent was obtained from all patients according to the declaration of Helsinki. Primary WM cells were obtained from bone marrow (BM) samples using CD19+ micro-bead selection (Milteny Biotec, Auburn, CA) with over 90% purity as previously described(19). Peripheral blood mononuclear cells (PBMCs) were obtained from healthy volunteers by Ficoll-Hypaque density sedimentation, and in some case CD19+ cells were selected using micro-bead selection.
Immunoblotting
WM cells were harvested and lysed using lysis buffer (Cell Signaling Technology, Beverly, MA) complemented with 5μM NaF, 2μM Na3VO4, 1μM PMSF, 5μg/mL leupeptin, and 5μg/mL aprotinin, following manufacturer instructions. For determination of the time dependent-activation of Src by SDF1, and the effect of AZD0530 on SDF1-induced activation of Src, cells were pre-starved for 3 hrs. Whole-cell lysates were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to PVDF membrane (Bio-Rad Laboratories, Hercules, CA) as previously described (19). All antibodies used for immune-blotting were purchased from Cell Signaling Technology), except the anti-β-actin and anti-tubulin which were purchased from Sigma, St Louis, MO.
Adhesion assays
To test adhesion to fibronectin we have used 96-well plates coated with fibronectin (EMD Biosciences, SanDiego, CA). Moreover, we have examined adhesion to both stromal and endothelial cells, in which a confluent monolayer of stromal or HUVEC cells was generated by plating (5×103) cells in a 96-well plate 24 hours before the experiment. HUVEC cells were activated with 10ng/mL TNF-α, for 1 hour before the experiment.
WM cells (BCWM1 and CD19+ from patient samples) were treated with 0 or 2 mM AZD0530 for 1hr; and cells (2 ×105/well) were incubated in the 96-well plates for 1 hr at 37°C. In adhesion to fibronectin experiments cells were activated with 30nM during incubation, and non-activated cells served as control. Moreover, BSA and poly-L-lysin coated wells were used as negative and positive controls, respectively. After incubation, wells were washed with PBS to remove all non-adherent cells, and Calcein-AM was added for 1 hour and the degree of fluorescence was measured using a spectrophotometer at excitation wavelength 485nm and emission wavelength 520nm.
Transwell and transendothelial Migration assays
Migration was determined using the transwell migration assay (Costar, NY), as previously described (20). WM cells (BCWM1 and CD19+ from patient samples) were treated with 0 or 2 μM AZD0530 in 1% FCS medium for 1hr, placed in the upper chambers, and were allowed to migrate to 0 or 30nM SDF-1 in 1% FCS medium, in the lower chamber. After 4 hours of incubation, cells that migrated to the lower chambers were counted using a Beckman Coulter cell counter (Beckman, Fullerton, CA).
Immunofluorescence
BCWM1 cells were treated with 0 or 2 mM AZD0530 in 1% FCS medium for 1hr, and then with 0 or 30nM SDF-1 for 1 min. Cell were then fixed with 2% Formalehyde in PBS for 30 min at RT, permeablized with Saponin 0.02% in PBS, stained with stained with (5μg/ml) phalloidin-Alexa-Fuor-568, spun onto slides, mounted, and analyzed by fluorescent microscopy, using an epifluorescence microscope (Nikon Eclipse E800; Nikon, Avon, MA) and a Photometrics Coolsnap CF color camera (Nikon, Lewisville, TX).
Cell cycle analysis
BCWM1 cells were treated overnight with increasing concentrations (0, 0.5, 1 and 2 μM) of AZD0530. Cell cycle were then fixed with methanol, RNA was degraded by RNAase, DNA was stained propidium iodide (PI) (5μg/mL, Sigma Chemical) and cells were analyzed by flow cytometry.
Cell viability test
BCWM1 cells, PBMCs and CD19+ cells from healthy volunteers (1× 106 cell/ml) were cultured with (0, 0.5, 1 and 2 μM) of AZD0530 for 24 hrs. Cell growth was assessed by measuring 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Chemicon International, Temecula, CA) dye absorbance.
Apoptosis
BCWM1 cells were treated overnight with increasing concentrations (0, 0.5, 1 and 2 μM) of AZD0530. Cells were then stained with Apo-2.7 labeled with Per-CP-Cy5.5 for 1hr on ice. Cells were then washed, resuspended in PBS and apoptosis was quantified by flow cytometry (Beckman Coulter Inc., CA).
Statistical analysis
Results were reported as the mean ± standard deviation for experiments done in three replicates samples and statistical significance was determined using Student T- test. The minimal level of significance was p < 0.05.
RESULTS
Expressed of Src tyrosine kinase in WM and the effect of SDF1 and AZD0530 on its phosphorylation
We first examined the expression of phosphorylated and total forms of Src kinase in WM patient samples and cell lines compared to expression in CD19+ cells obtained from the BM of healthy donors. Figure 1A shows that Src was over expressed in WM patients and in BCWM1 compared to CD19+ cells from healthy donors, and that the levels of phosphorylation of Src correlated with the expression of total Src. Figure 1B shows that AZD0530 inhibited the basal phosphorylation of Src in BCWM1 cells in a dose-response manner, and that the dose of 2μM led to a complete inhibition of basal phosphorylation of Src.
Figure 1. Expressed of Src tyrosine kinase in WM and the effect of SDF1 and AZD0530 on its phosphorylation.
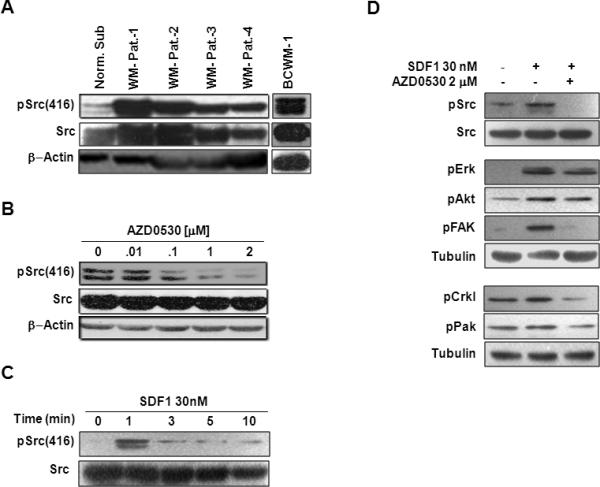
(A) Immunoblotting for Src and pSrc expression in cell from four WM patients, BCWM1 cell line and CD19+ cells from healthy donors. (B) The effect of AZD0530 on Basal activation of Src in BCWM1. (C) Time dependent activation of Src by SDF1 in BCWM1. (D) The effect of AZD0530 on the SDF1-induced cytoskeletal-signaling. β-actin and tubulin served as loading control.
Figure 1C shows that activation of BCWM1 cells with SDF1 induced phosphorylation of Src in a time-dependent manner, and that the highest level of activation was achieved after 1 min of activation. Figure 1D shows that the SDF1-induced phosphorylation of Src and other cytoskeletal-signaling-related proteins such as ERK, AKT, FAK, Crkl and PAK, which were all inhibited by AZD0530.
The role of Src in regulating adhesion and migration induced by SDF1
Figures 2A and B show the adhesion to fibronectin of BCWM1 cells and cell from three WM patients, respectively. SDF1 increased the adhesion of WM cells to fibronectin, and AZD0530 inhibited the SDF1-induced adhesion to fibronectin. Moreover, Figure 2C shows that AZD0530 inhibited the adhesion of BCWM1 cells to both stromal and endothelial cells (HUVEC cells).
Figure 2. The role of Src in adhesion of WM to BM components.
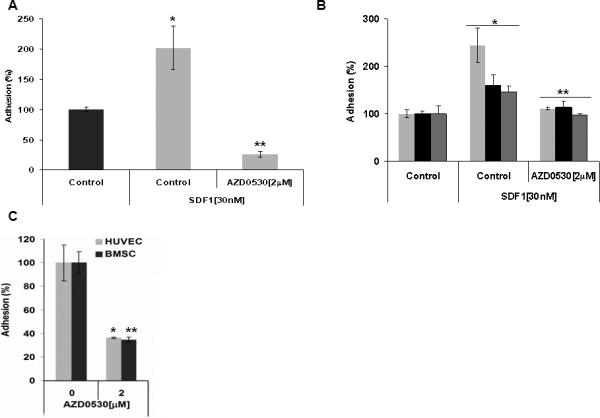
The effect of AZD0530 on (A) SDF1-induced adhesion of BCWM1 to fibronectin, (B) SDF1-induced adhesion of cells from three WM patients to fibronectin, and (C) adhesion of BCWM1 to BM stromal cells and endothelial cells.
(A) *p<0.030, **p<0.013 (B) Patient-1 *p<0.010, **p<0.018; Patient-2 *p<0.029, **p<0.050), Patient-3 *p< 0.046, **p< 0.0160 (C) *p<0.026, **p<0.021.
Similarly, Figures 3A and B show chemotaxis of BCWM1 and cells from three WM patients, respectively. SDF1 induced chemotaxis of WM cells, and AZD0530 inhibited the SDF1-induced chemotaxis in WM cells. To further confirm that the effect of CXCR4/SDF-1 axis was regulated by Src, we have tested the effect of AZD0530 in the presence or absence of the CXCR4 inhibitor AMD3100. Figure 3C shows that AMD3100 and AZD0530 had similar inhibitory activity on migration of WM cells, and their combination had no additive effect.
Figure 3. The role of Src in SDF1-induced chemotaxis of WM to BM components.
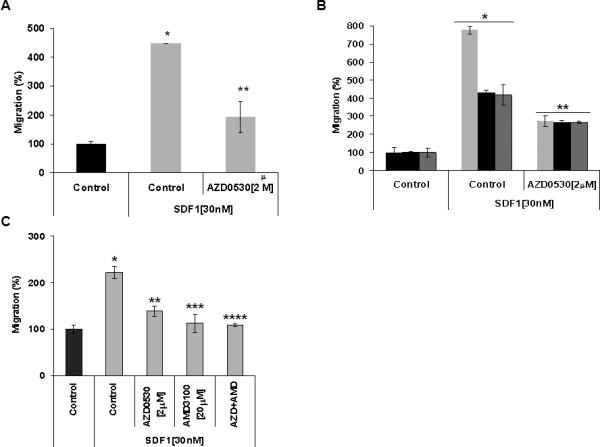
The effect of AZD0530 on SDF1-induced chemotaxis of (A) BCWM1 and (B) cells from three WM patients. (C) The effect of AZD0530 on SDF1-induced chemotaxis of BCWM1 in the presence and absence of the CXCR4 inhibitor AMD3100
(A) *p<0.035, **p<0.013 (B) Patient-1 *p<0.002, **p<0.003; Patient-2 *p<0.002, **p<0.008; Patient-3 *p< 0.015, **p< 0.050 (C) *p<0.007, **p<0.043, ***p<0.009, ****p<0.006.
Both adhesion and chemotaxis are known to depend on actin metabolism and polymerization. Figure 4 shows that SDF-1 (30nM for 1 minute) induced a significant increase in the polymerization of actin (F-actin) in BCWM.1, this effect was significantly inhibited by AZD0530.
Figure 4. The role of Src in SDF1-induced actin polymerization.
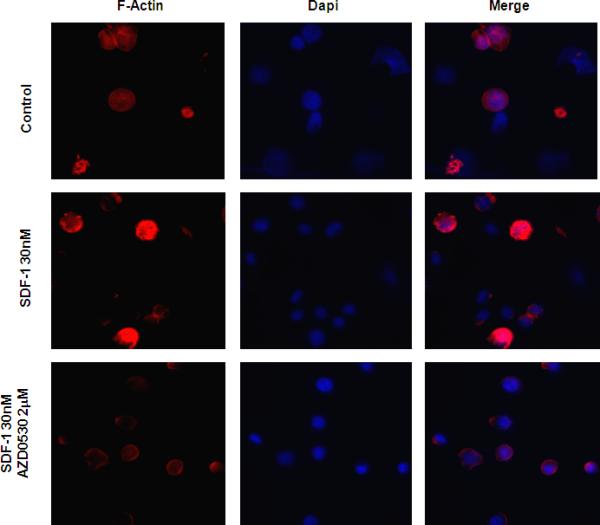
Representative immune-fluorescence images showing that AZD0530 inhibited the SDF1-induced polymerization of actin in BCWM1 cells. Left column: polymerized actin detected by phalloidin (red), middle column: DAPI staining of cell nuclei (blue), and right column: the merge of the actin polymerization and nuclei images.
Src regulates cell cycle in WM
pSrc is implicated in inducing proliferation in many malignancies(1, 12). We therefore investigated the role of AZD0530 on the cell cycle of WM. Figure 5A shows that AZD0530 induced G1-arrest in a dose-dependent manner. Moreover, Figure 5B shows that inhibition of Src with AZD0530 induced inhibition of other kinases important for cell proliferation such as ERK, AKT and S6R. Moreover, it inhibited proteins involved in cell cycle transmission from G1 to S phase such as the cyclin dependent kinases CDK2 and CDK6, and increased the activity of the cyclin dependent kinase inhibitor p27.
Figure 5. The role of Src in cell cycle regulation.
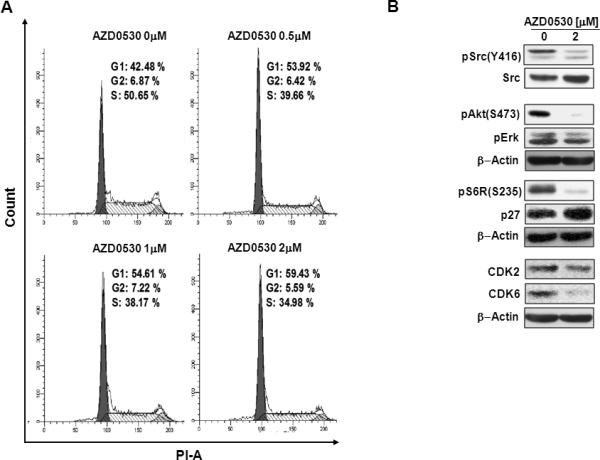
(A) The effect of increasing concentrations of AZD0530 on the cell cycle of BCWM1 cells, showing dose-dependent G1-arrest. (B) The effect of AZD0530 on protein related to cell proliferation and cell cycle in BCWM1 cells detected by immune-blotting, showing a decrease of the activation proliferative and cell cycle promoting of proteins, and an increase of cell cycle inhibitory proteins.
Cytotoxicity of AZD0530
Supplementary Figure 1A shows AZD0530 did not significantly inhibit proliferation or induce apoptosis at the concentrations used to inhibit pSrc activity, and inhibit adhesion of WM cells. Treatment with 2 μM AZD0530 for 24hrs induced only 30% of inhibition of proliferation and 20% of apoptosis in BCWM1 cells. Moreover, Supplementary Figures 1B and C show that no cytoxic effect of AZD0530 was observed on either PBMCs or CD19+ cells from healthy donors, even at concentration that completely inhibited pSrc activity.
DISCUSSION
WM is characterized by widespread involvement of the bone marrow in all patients, indicating homing and adhesion of the malignant cells to specific niches in the bone marrow, which provide a protective environment for the survival and proliferation of these cells (21). We have previously showed that the migration and adhesion of WM cells to the bone marrow niches is dependent on the SDF-1/CXCR4 axis and that inhibition of this axis regulates adhesion through a direct interaction of the CXCR4 and VLA-4 receptors (17). Src tyrosine kinase is known to regulate cell adhesion and migration, however, its role in SDF-1-induced migration and adhesion has not been previously studied in B-cell malignancies. We therefore sought to investigate the role of the tyrosine kinase Src in regulating adhesion and migration in WM, and to delineate the pathways regulated by Src through the SDF1/CXCR4 axis.
In this study, we first delineated the role of Src kinase and downstream pathways through the differential response of cell lines and patient samples. We found that Src was over expressed in WM patient samples and in the WM cell line BCWM1 compared to CD19+ cells from healthy donors. This indicates a potential role of Src in the invasiveness and metastasis of WM cells.
To investigate the role of Src in WM we used the specific Src/Abl kinase inhibitor AZD0530. Previous studies in solid tumors and hematological malignancies, such as leukemias, have shown that AZD0530 is a specific Src/Abl tyrosine kinase inhibitor, and that it regulates proliferation, invasion and metastasis in these malignancies (22-26). AZD0530 is currently being tested in phase II clinical trials. AZD0530 was shown to inhibit the basal activation of Src in BCWM1, as well as inhibition of the SDF1-induced activation of Src and other cytoskeletal-realated protein and polymerization of actin indicating an important role of Src in the signaling cascade downstream of CXCR4 leading to an increase of migration and adhesion of WM cells.
Functionally, in both BCWM1 cell line and in cells from WM patients , we found that Src was involved in adhesion of MM cells to the different components of the BM such as BM stromal cells, endothelial cells and fibronectin that represents the extracellular matrix. Moreover, it inhibited the chemotactic effect of SDF1 which leads to increased infiltration of WM to the BM. We further confirmed that the effect SDF1/CXCR4 is Src dependent, as we showed that inhibiting either CXCR4 by AMD3100 or inhibition of Src resulted in similar effects of inhibition of the chemotaxis. In addition, the combination of the two inhibitors did not have any additive or synergistic effect suggesting that both CXCR4 and Src are in the same signaling cascade, and inhibiting either of them will have similar activity.
Moreover, we showed that Src was involved in controlling cell-cycle in WM, in which AZD0530 induced G1-arrest, and it partially inhibited signaling cascades involved in cell-proliferation such as ERK and AKT, which led to inhibition of cell-cycle proteins. However, complete inhibition of Src by AZD0530 did not inhibit cell survival neither in WM cells nor in normal PBMCs and CD19+ cells.
Together, these studies indicate that the Src tyrosine kinase regulates SDF1-induced adhesion and migration of WM cells, and its inhibition by AZD0530 reduced those effects. These studies indicate that AZD0530 can be used in the future to inhibit dissemination of WM cells. Moreover, the lack of cytotoxicity of AZD0530 on normal cells suggests it as a safe drug to be used in combination with other chemotherapies to improve patient outcome in WM.
Supplementary Material
Supplementary Figure 1. The role of Src in survival of WM and normal cells.
The effect of increasing concentrations of AZD0530 on (A) cell proliferation and apoptosis of BCWM1 cells detected by flow cytometry, (B) survival of normal PBMCs, and (C) survival of normal CD19+ cells. This figure shows a minimal cytotoxic effect of AZD0530 on WM cells and no cytotoxic effect on normal PBMCs and CD19+ cells.
Acknowledgment
We would like to acknowledge the contribution of Jennifer Stedman in reviewing and editing this manuscript.
Supported in part by R21CA126119-01, the International Waldenstrom Macroglobulinemia Foundation, The Kirsch Laboratory for Waldenstrom, and R01CA125690-01.
Footnotes
Statement of Translational Relevance.
In this study, we tested the role of a specific Src kinase inhibitor on adhesion, migration and survival of Waldenstrom Macroglobulinemia cells. The use of the Src inhibitor AZD0530 led to significant inhibition of adhesion, migration and cytoskeletal signaling induced by SDF1, as well as cell cycle. Taken together, these studies delineate the role of Src kinase activity in WM and provide the framework for future clinical trials using Src inhibitors in combination other therapeutic agents to improve the outcome of patients with WM.
References
- 1.Biscardi JS, Tice DA, Parsons SJ. c-Src, receptor tyrosine kinases, and human cancer. Adv Cancer Res. 1999;76:61–119. doi: 10.1016/s0065-230x(08)60774-5. [DOI] [PubMed] [Google Scholar]
- 2.Homsi J, Cubitt C, Daud A. The Src signaling pathway: a potential target in melanoma and other malignancies. Expert Opin Ther Targets. 2007;11:91–100. doi: 10.1517/14728222.11.1.91. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez RH, Kantarjian HM, Cortes JE. The role of Src in solid and hematologic malignancies: development of new-generation Src inhibitors. Cancer. 2006;107:1918–29. doi: 10.1002/cncr.22215. [DOI] [PubMed] [Google Scholar]
- 4.Alper O, Bowden ET. Novel insights into c-Src. Curr Pharm Des. 2005;11:1119–30. doi: 10.2174/1381612053507576. [DOI] [PubMed] [Google Scholar]
- 5.Ishizawar R, Parsons SJ. c-Src and cooperating partners in human cancer. Cancer Cell. 2004;6:209–14. doi: 10.1016/j.ccr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Gauld SB, Cambier JC. Src-family kinases in B-cell development and signaling. Oncogene. 2004;23:8001–6. doi: 10.1038/sj.onc.1208075. [DOI] [PubMed] [Google Scholar]
- 7.Parsons SJ, Parsons JT. Src family kinases, key regulators of signal transduction. Oncogene. 2004;23:7906–9. doi: 10.1038/sj.onc.1208160. [DOI] [PubMed] [Google Scholar]
- 8.Ram PT, Iyengar R. G protein coupled receptor signaling through the Src and Stat3 pathway: role in proliferation and transformation. Oncogene. 2001;20:1601–6. doi: 10.1038/sj.onc.1204186. [DOI] [PubMed] [Google Scholar]
- 9.Resh MD. The ups and downs of SRC regulation: tumor suppression by Cbp. Cancer Cell. 2008;13:469–71. doi: 10.1016/j.ccr.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Ingley E. Src family kinases: regulation of their activities, levels and identification of new pathways. Biochim Biophys Acta. 2008;1784:56–65. doi: 10.1016/j.bbapap.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Summy JM, Gallick GE. Treatment for advanced tumors: SRC reclaims center stage. Clin Cancer Res. 2006;12:1398–401. doi: 10.1158/1078-0432.CCR-05-2692. [DOI] [PubMed] [Google Scholar]
- 12.Chen J. Is Src the key to understanding metastasis and developing new treatments for colon cancer? Nat Clin Pract Gastroenterol Hepatol. 2008;5:306–7. doi: 10.1038/ncpgasthep1141. [DOI] [PubMed] [Google Scholar]
- 13.Bayes M, Rabasseda X. Gateways to clinical trials. Methods Find Exp Clin Pharmacol. 2008;30:67–99. [PubMed] [Google Scholar]
- 14.Kopetz S, Shah AN, Gallick GE. Src continues aging: current and future clinical directions. Clin Cancer Res. 2007;13:7232–6. doi: 10.1158/1078-0432.CCR-07-1902. [DOI] [PubMed] [Google Scholar]
- 15.Ghobrial IM, Gertz MA, Fonseca R. Waldenstrom macroglobulinaemia. Lancet Oncol. 2003;4:679–85. doi: 10.1016/s1470-2045(03)01246-4. [DOI] [PubMed] [Google Scholar]
- 16.Treon SP, Dimopoulos M, Kyle RA. Defining Waldenstrom's macroglobulinemia. Semin Oncol. 2003;30:107–9. doi: 10.1053/sonc.2003.50081. [DOI] [PubMed] [Google Scholar]
- 17.Ngo HT, Leleu X, Lee J, et al. SDF-1/CXCR4 and VLA-4 interaction regulates homing in Waldenstrom macroglobulinemia. Blood. 2008;112:150–8. doi: 10.1182/blood-2007-12-129395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ditzel Santos D, Ho AW, Tournilhac O, et al. Establishment of BCWM.1 cell line for Waldenstrom's macroglobulinemia with productive in vivo engraftment in SCID-hu mice. Exp Hematol. 2007;35:1366–75. doi: 10.1016/j.exphem.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 19.Leleu X, Eeckhoute J, Jia X, et al. Targeting NF-{kappa}B in Waldenstrom macroglobulinemia. Blood. 2008;111:5068–77. doi: 10.1182/blood-2007-09-115170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alsayed Y, Ngo H, Runnels J, et al. Mechanisms of regulation of CXCR4/SDF-1 (CXCL12)-dependent migration and homing in multiple myeloma. Blood. 2007;109:2708–17. doi: 10.1182/blood-2006-07-035857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vijay A, Gertz MA. Waldenstrom macroglobulinemia. Blood. 2007;109:5096–103. doi: 10.1182/blood-2006-11-055012. [DOI] [PubMed] [Google Scholar]
- 22.Schweppe RE, Kerege AA, French JD, et al. Inhibition of Src with Azd0530 Reveals the Src-Focal Adhesion Kinase Complex as a Novel Therapeutic Target in Papillary and Anaplastic Thyroid Cancers. J Clin Endocrinol Metab. 2009;94:2199–203. doi: 10.1210/jc.2008-2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Purnell PR, Mack PC, Tepper CG, et al. The Src Inhibitor AZD0530 Blocks Invasion and May Act as a Radiosensitizer in Lung Cancer Cells. J Thorac Oncol. 2009;4:448–54. doi: 10.1097/JTO.0b013e31819c78fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saad F. Src as a therapeutic target in men with prostate cancer and bone metastases. BJU Int. 2009;103:434–40. doi: 10.1111/j.1464-410X.2008.08249.x. [DOI] [PubMed] [Google Scholar]
- 25.Rucci N, Susa M, Teti A. Inhibition of protein kinase c-Src as a therapeutic approach for cancer and bone metastases. Anticancer Agents Med Chem. 2008;8:342–9. doi: 10.2174/187152008783961905. [DOI] [PubMed] [Google Scholar]
- 26.Hiscox S, Morgan L, Green TP, et al. Elevated Src activity promotes cellular invasion and motility in tamoxifen resistant breast cancer cells. Breast Cancer Res Treat. 2006;97:263–74. doi: 10.1007/s10549-005-9120-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. The role of Src in survival of WM and normal cells.
The effect of increasing concentrations of AZD0530 on (A) cell proliferation and apoptosis of BCWM1 cells detected by flow cytometry, (B) survival of normal PBMCs, and (C) survival of normal CD19+ cells. This figure shows a minimal cytotoxic effect of AZD0530 on WM cells and no cytotoxic effect on normal PBMCs and CD19+ cells.


