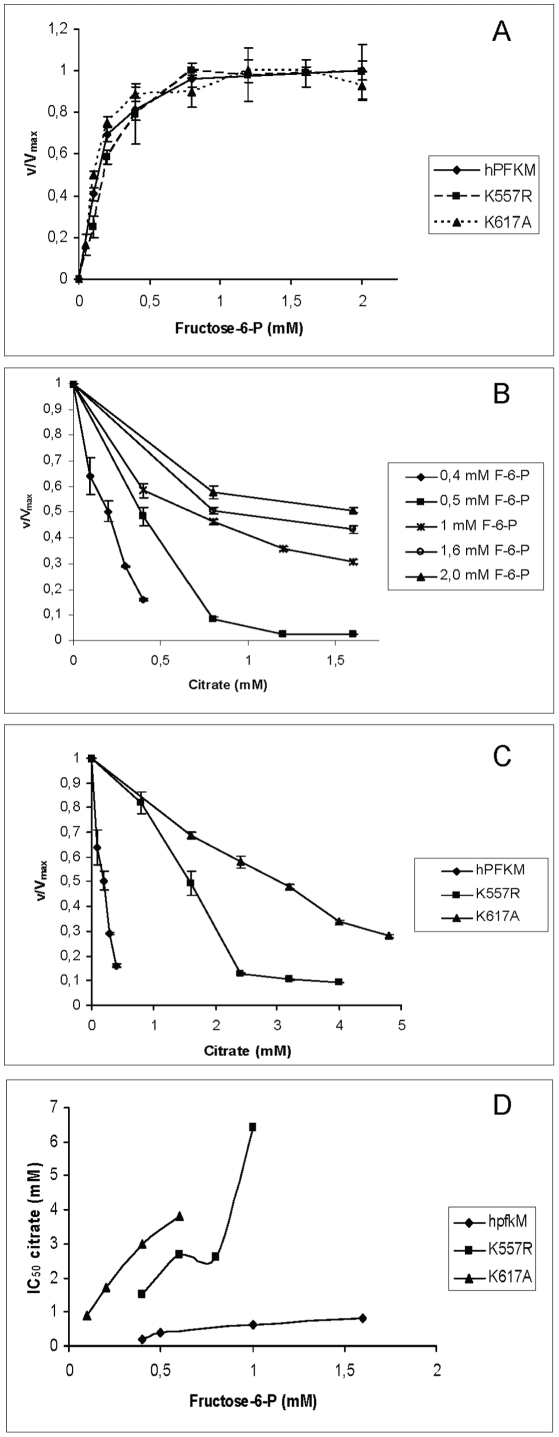
Figure 3. Kinetic measurements of recombinant human PFK-M and mutant forms of PFK-M.
A. Fructose-6-phosphate (F6P) saturation curves for the native human and mutant forms of PFK-M. Measurements were carried out at pH 7.8 in a buffer containing 5 mM Mg2+ and 0.5 mM ATP. Activities are expressed as a ratio of enzyme activity (v) at a specific substrate concentration to the activity detected at saturating F6P concentration (Vmax). Data are presented as means ± standard deviation. B. Citrate inhibition of the native human PFK-M measured at different fructose-6-phosphate (F6P) concentrations. The assay was performed at pH 7.8 in a buffer containing 5 mM Mg2+ and 0.5 mM ATP. Data are presented as means ± standard deviation. C. Citrate inhibition of the native and mutant forms of human PFK-M. All measurements were conducted at 0.4 mM F6P. The assay was carried out at pH 7.8 in the presence of 5 mM Mg2+ and 0.5 mM ATP. Activities are expressed as a ratio of activity detected in the presence of citrate to activity measured without citrate in the system. Data are presented as means ± standard deviation. D. IC50 values for citrate inhibition of the native and mutant forms of human PFK-M measured at increasing concentrations of F6P. The assay was carried out at pH 7.8 in the presence of 5 mM Mg2+ and 0.5 mM ATP. Data were obtained by determining the citrate concentration that caused inhibition of the wild type and mutated forms of PFK-M by 50%. Mean values of at least three independent measurements are reported.