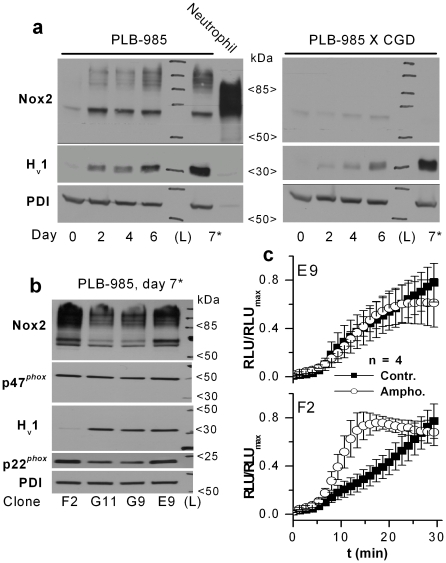
Figure 6. The functionally coupled Hv1 and Nox2 are induced in parallel, but largely independently during granulocytic differentiation in PLB-985 cells.
(a) Normal Hv1 expression can be induced in the absence of normal Nox2 level. For WB total cell lysates of 106 cells were loaded each lane. Samples of PLB-985 cells were prepared before (0) and 2, 4, 6 days after inducing differentiation with 0.5% DMFA. In a separate sample (7*) cells were differentiated for 7 days, and DMFA treatment was applied in low-serum culture medium (0.5% v/v) to increase the differentiation pressure. Different anti-Nox2 labeled bands correspond to different glycozilation states of the 65 kDa Nox2 protein. (b) The normal expression of different phox subunits (Nox2, p22phox and p47phox) is not disturbed by strongly reduced Hv1 expression in differentiated PLB-985 cells. (c) Amongst differentiated PLB-985 clones amphotericin B amplifies superoxide production (2.92±0.48 times at 15 min, p<0.05, Mann-Whitney U test) only in clone F2, in which Hv1 expression is strongly diminished. No significant change in superoxide production was detected in E9, G9 and B7 clones in the presence of amphotericin B. Diogenes™ reagent was used to detect the extracellular release of superoxide. Cells were preincubated for 15 min in a 1∶1 mixture of Diogenes™ and H-medium with or without 10 µg/ml amphotericin B. At time point 0 cells were activated by 200 nM PMA. Negligible Diogenes™ luminescence could be detected in PMA-treated, non-differentiated PLB-985 clones and during the preincubation period (not shown).