Fig. 2.
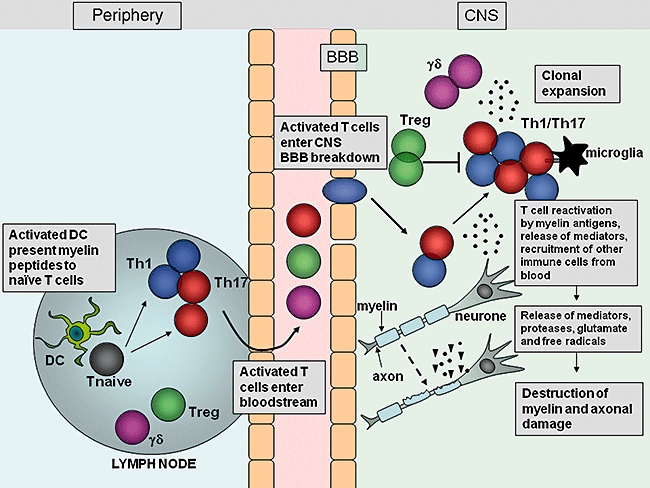
Migration and effector function of T cells in the central nervous system (CNS) during experimental autoimmune encephalomyelitis (EAE). After immunization with myelin antigens, complete Freund's adjuvant (CFA) and pertussis toxin, dendritic cells (DC) are activated in the lymph nodes by Toll-like receptor (TLR) agonists within the mycobacterium tuberculosis component of CFA, and present myelin antigen to naive T cells. The activated myelin-specific T cells enter the bloodstream and traffic to and enter the CNS. Breakdown of the blood–brain barrier (BBB) occurs, allowing recruitment of other inflammatory cells into the CNS. T cells entering the CNS encounter their cognate myelin antigens and become reactivated by local APC. T cells expand and release inflammatory mediators which help recruit other immune cells to the site of inflammation. Activation of local microglial cells and infiltrating cells results in production of proteases, glutamate, reactive oxygen species and other cytotoxic agents which promote myelin breakdown. Damage to the myelin sheath surrounding axons is followed by axonal damage and neurological impairment.
