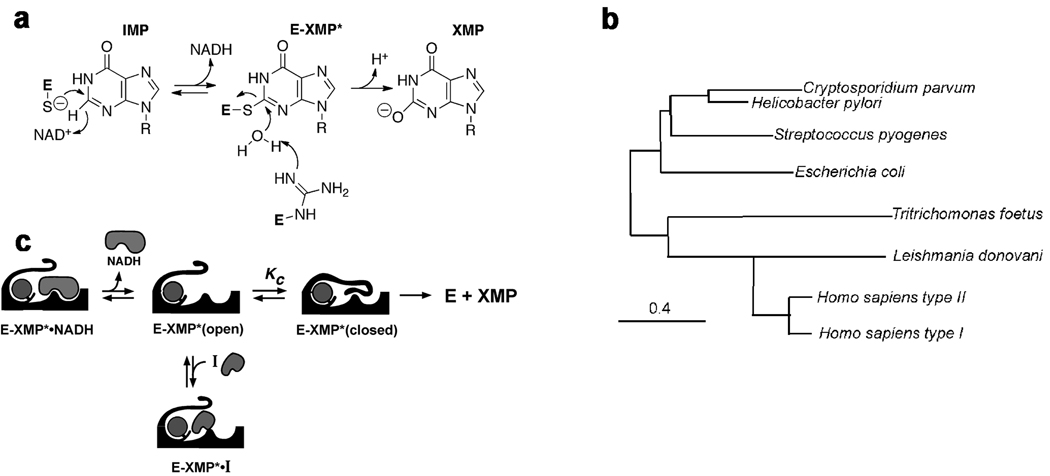
Figure 1. The IMPDH reaction.
a. Chemical mechanism: a conserved Cys attacks C2 of IMP and hydride is transferred to NAD+ producing the covalent intermediate E-XMP*. E-XMP* is hydrolyzed with a conserved Arg residue acting as a general base to produce XMP. b. The hydride transfer reaction proceeds in an open enzyme conformation. After NADH departs, a mobile flap folds into the NAD site, carrying the catalytic Arg into the active site. Inhibitors compete with the flap, so the equilibrium between open and closed states is a determinant of inhibitor affinity. c. Phylogenetic tree of IMPDHs. Constructed as described in (Min, et al., 2008).