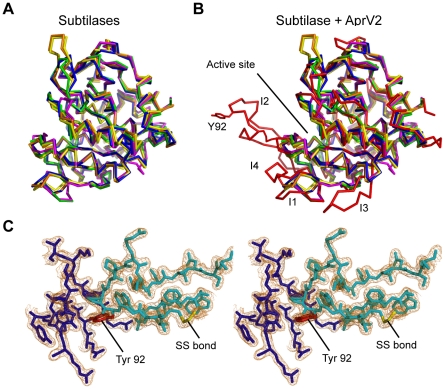
Figure 6. AprV2 contains a novel disulfide tethered loop.
(A) Overlay of the crystal structures of AkP (magenta; 1DBI), thermitase (green; 1THM), Carlsburg subtilisn (yellow; 1AF4), BPN' (orange; 1SUP) and savinase (blue; 1SVN). (B) Overlay of the crystal structures of AprV2 (red) with AkP, thermitase, Carlsburg subtilisn, BPN' and savinase (coloured as in (a)). The structures were superimposed using the A chains only and are shown as a Cα trace. The I1, I2, I3 and I4 loops are labelled. Tyr 92 in AprV2 and Arg 92 are shown in stick representation and labelled. (C) Stereo view of a 2|Fo|−|Fc| electron density map depicting the disulfide tethered I2 loop of the AprV2 protease. The map is contoured at 1.2 σ. Water molecules have been removed for clarity. The conformation of this loop (cyan) is stabilised by inter and intramolecular contacts with molecules generated by symmetry coloured blue. The alignments in (A) and (B) were generated using MUSTANG [52]. Secondary structure elements were calculated using stride [53]. The figure was prepared using PyMol [51].