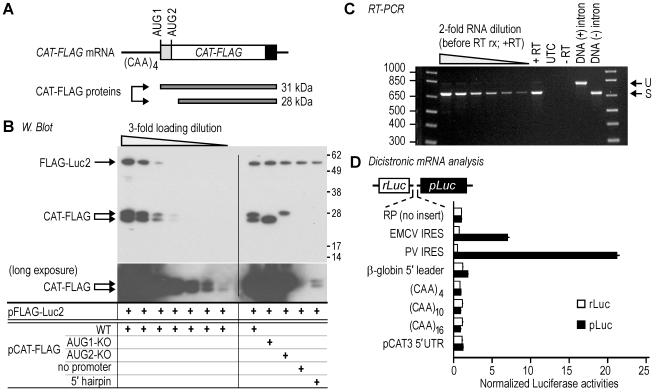
Figure 1. Translation of a synthetic CAT-FLAG mRNA initiates at two AUG codons in a cap-dependent manner.
A. The CAT-FLAG mRNA used in this study is indicated schematically. It contains four CAA repeats in the 5′ leader and two in-frame AUG codons. FLAG epitopes are indicated by the black bar. This mRNA encodes two proteins with predicted molecular weights of 31 and 28 kDa. B. Western blot analysis. COS-7 cells were transiently cotransfected with plasmid constructs that express the (CAA)4 CAT-FLAG and the control FLAG-Luc2 mRNAs. The wild-type CAT-FLAG construct (WT) contains both AUG codons; AUG1-KO lacks AUG1 (the U has been deleted); and AUG2-KO lacks AUG2 (mutated to AAG). The no promoter construct lacks SV40 promoter/enhancer sequences and the 5′ hairpin construct contains an inverted repeat sequence at the 5′ terminus of the mRNA. A longer film exposure of the blot is shown for the CAT-FLAG protein. C. RT-PCR analysis of (CAA)4 CAT-FLAG mRNA from COS-7 cells transfected with a plasmid expressing this mRNA (+RT). Control reactions used RNA from untransfected cells (UTC) or did not contain reverse transcriptase (-RT). Size controls for PCR products of unspliced (U) or correctly spliced (S) mRNAs were amplified from plasmids containing an intron (DNA (+) intron) or lacking an intron (DNA (-) intron), using the same PCR conditions in parallel. Two-fold dilutions of the (CAA)4 CAT-FLAG RNA sample were reverse-transcribed prior to PCR amplification. D. Renilla/Photinus dual luciferase dicistronic analysis. The 5′ leader sequences were tested in the intercistronic region of the dicistronic mRNA. Intercistronic sequences in the parent vector (RP) and the β-globin 5′ leader were used as negative controls for IRES and promoter activities; the EMCV and PV 5′ leaders were used as positive controls for IRES activity. Renilla luciferase (rLuc) activities are indicated by white bars; Photinus luciferase (pLuc) activities are indicated by black bars. Luciferase activities were normalized to 1.0 for activities obtained with the RP construct. Three independent experiments were performed for final quantification; error bars indicate standard deviations.