Figure 2.
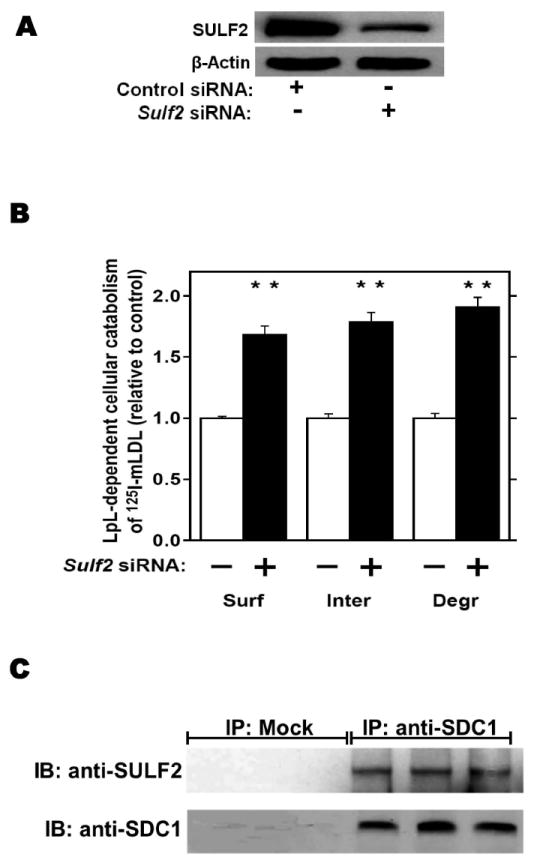
Endogenous SULF2 strongly inhibits the catabolism of model remnant lipoproteins by cultured liver cells.
McArdle hepatoma cells were pre-incubated for 24h with 100nM non-target (Control) or Sulf2 siRNA, as indicated, followed by an additional 44h at 37°C. Cells were then incubated for 4h at 37°C with model remnant lipoproteins that we prepared by combining 125I-labeled methylated LDL with lipoprotein lipase (LpL), a molecule that bridges between lipoproteins and HSPGs (background values were assessed in the absence of LpL). Panel A: Immunoblots of cellular homogenates, using anti-SULF2 antibodies or, as a loading control, anti-ß-actin antibodies. Panel B: LpL-dependent cellular catabolism of model remnant lipoproteins, shown as surface binding (Surf), internalization (Inter), and degradation (Degr), normalized to control values from cells treated with the nontarget siRNA (means±SEMs, n=3; the non-normalized control values were 194±2.9, 605±20.6, and 112±4.3 ng/mg, respectively). **, P<0.01 by the two-tailed Student’s t-test. Displayed are data from a representative experiment from a total of four independent knock-down studies. Panel C: Coimmunoprecipitation of SULF2 with the syndecan-1 HSPG. McArdle hepatocytes were extracted into NP-40 and subjected to immunoprecipitation with nonimmune IgG (IP: Mock) or anti-syndecan-1 IgG (IP: anti-SDC1), followed by electrophoretic separation. Displayed are immunoblots that were performed to detect SULF2 (IB: anti-SULF2), and then the same blots were stripped and reprobed to detect syndecan-1 (IB: anti-SDC1).
