Abstract
Oral reading is a complex skill involving the interaction of orthographic, phonological, and semantic processes. Functional imaging studies with non-impaired adult readers have identified a widely distributed network of frontal, inferior parietal, posterior temporal, and occipital brain regions involved in the task. However, while functional imaging can identify cortical regions engaged in the process under examination, it cannot identify those brain regions essential for the task. The current study aimed to identify those neuroanatomical regions critical for successful oral reading by examining the relationship between word and nonword oral reading deficits and areas of tissue dysfunction in acute stroke. We evaluated 91 patients with left hemisphere ischemic stroke with a test of oral word and nonword reading, and magnetic resonance diffusion-weighted and perfusion-weighted imaging, within 24–48 hours of stroke onset. A voxel-wise statistical map showed that impairments in word and nonword reading were associated with a distributed network of brain regions, including the inferior and middle frontal gyri, the middle temporal gyrus, the supramarginal and angular gyri, and the middle occipital gyrus. In addition, lesions associated with word deficits were found to be distributed more frontally, while nonword deficits were associated with lesions distributed more posteriorly.
Keywords: Oral Reading, Neuroanatomical Localisation, Acute Stroke
Oral reading is a learned linguistic skill involving the complex interaction of orthographic, phonological, and semantic processes. Numerous studies have attempted to identify the brain regions associated with reading in an attempt to uncover more about the functional architecture of the brain, and the nature of the representations and processes involved in this highly specialised skill. Functional imaging studies with skilled adult readers have identified a widely distributed network of brain regions, including the occipital cortex (notably the extrastriate visual cortex), the occipitotemporal cortex (including the fusiform gyrus), posterior temporal regions, inferior parietal regions (notably the supramarginal gyrus), inferior frontal regions (notably pars triangluaris and opercularis), and premotor regions (for meta-analyses see Joseph, Noble, & Eden, 2001; Schlagger & McCandliss, 2007; Turkeltaub, Eden, Jones, & Zeffiro, 2002).
Although various cognitive models of reading differ in the implementation of component processes, many implicate two different distinct (but in some models, interacting) strategies for reading: a lexical or semantic route used for the reading of words (particularly those with irregular spelling-sound correspondences), in which orthography is mapped onto phonology via access to semantic information; and a sublexical or phonological route used for the reading of unfamiliar words or nonwords, involving the orthographic analysis of letter strings into graphemic units, and the subsequent mapping of these graphemes onto phonemic units (see e.g., Coltheart, Rastle, Perry, Ziegler, & Langdon, 2001). In relation to specific processes implicated in such models, functional imaging studies have implicated occipital and occipitotemporal regions, particularly the fusiform gyrus, in visual letter/word form processing, inferior parietal and superior temporal in the conversion of orthography to phonology, and inferior frontal and premotor regions, in phonological recoding and output for production (Joseph et al., 2001). Other researchers have noted that the lexical-semantic reading processes appear to be associated with posterior middle/inferior temporal regions and the triangular part of the inferior frontal gyrus, while phonological processing appears associated with the superior temporal gyrus, supramarginal gyrus, and the opercular part of the inferior frontal gyrus (Jobard, Crivello, & Tzourio-Mazoyer, 2003; Salmelin & Kujala, 2006). Thus there appears to be some subdivision and specialisation of the functional architecture of the reading network.
However, other models of reading do not specify separate mechanisms for reading words and nonwords (or unfamiliar words), but a single set of distributed phonological and orthographic (and, in some models, semantic) representations or nodes that are activated in parallel to compute word or nonword output (Harm & Seidenberg, 2004; Plaut, McClelland, Seidenberg, & Patterson, 1996; Plaut, 1997; Seidenberg & McClelland, 1989). This single parallel distributed process is sensitive to statistical relationships between orthographic and phonological patterns that arise from repeated word exposure and associated output. These models would predict that a single network of neural regions is engaged and necessary for word and nonword reading, but the precise pattern and intensity of activation might differ for words and nonwords (or highly familiar words and less familiar words). Indeed, most functional imaging studies do show activation of overlapping if not identical regions during reading of words and nonwords, although there may be some differences in the degree to which different types of stimuli depend on relatively localised components of the single reading process. One review of nine functional imaging studies of reading concluded that there is some evidence for proposing the following functionally specialised regions: (i) primary motor cortex (Brodmann’s Area – BA – 4), supplementary motor area (BA 6), and medial cerebellum for motor speech production; (ii) anterior superior temporal cortex (BAs 22 and 41) bilaterally for auditory feedback activated by the reader’s own vocalization; (iii) left posterior temporal cortex (BA 22) for acoustically based phonological analysis; (iv) left inferior frontal and anterior insular cortex for articulatorily based phonological analysis; (v) the borderzone between superior and middle temporal gyrus (BA 22/21) in semantic analysis; and (vi) left occipital and occipitotemporal/fusiform cortex (BAs 18, 19, and 37) for visual analysis specific to word and word-like stimuli (Fiez & Petersen, 1998). Note semantic analysis would only be required for word processing, but BA 22 would nevertheless be activated for acoustically based phonological analysis for nonwords. Further discussion of the difficulty distinguishing between single and dual route reading models on the basis of functional imaging studies is provided by Fiez and Petersen (1998).
Despite the presence of consistent similarities across studies in the brain regions implicated in normal word reading, there are numerous discrepancies across functional imaging studies, which have led to considerable debate regarding the involvement of certain brain areas. For example, various studies have implicated the angular gyrus in lexical-semantic reading processes (e.g., Binder et al., 2003), phonological reading processes (e.g., Pugh et al., 2001), or have failed to find any significant involvement of this region at all (Turkeltaub et al., 2002). As has been noted by several researchers, one key shortcoming of functional imaging studies is that while the methodology can identify cortical regions that are engaged in the process under examination, it cannot identify which brain regions are essential for the task (Davis, Hillis, Bergey, & Ritzl, 2007).
One methodology which can provide complementary evidence regarding the critical involvement of specific brain regions in reading words and nonwords is the study of acquired reading deficits following lesions to specific cortical regions. Acquired dyslexia is a common language impairment following left hemisphere brain damage, and has been studied extensively in an attempt to understand the specific processes involved in both normal and impaired reading (see Bub, 2003, for a review of acquired dyslexias). Lesions associated with acquired reading impairments involve a large network of left hemisphere regions including inferior frontal, temporo-parietal, and occipital regions (Lambon Ralph & Graham, 2000; Price & Mechelli, 2005). Specifically, these studies have implicated occipito-temporal regions, particularly the fusiform gyrus, in orthographic processing (implicated in pure alexia; see Leff et al., 2001); inferior frontal and temporo-parietal regions with phonological processing (implicated in phonological dyslexia; see Hamilton & Coslett, 2008; Rapcsak et al., 2009), and a fronto-temporo-parietal network in lexical-semantic processing (implicated in deep dyslexia; see Laine, Niemi, Niemi, & Koivuselka-Sallinen, 1990). Thus, the brain regions implicated by examination of lesions associated with acquired reading deficits appear similar to those identified by the functional imaging studies of non-impaired readers. However, like the functional imaging studies with unimpaired readers, there are inconsistencies between lesion studies, and different lesions have been found to produce to similar deficits. For example, both anterior and posterior perisylvian brain lesions have been found to be implicated in acquired phonological dyslexia (Rapcsak et al., 2009).
Very few lesion studies have examined the reading performance of a large number of individuals following brain damage, with the majority adopting a case study or case series approach, with one or a few patients with chronic stroke. In relation to neuroanatomical localisation, such studies suffer from the fact that the vast majority of lesions in these chronic stroke patients are large, encompassing a number of brain structures, making it difficult to identify the specific brain regions implicated in the observed reading deficits. In addition, such studies are unable to demonstrate that damage to the same cortical region will consistently produce similar deficits across all patients. One important problem with previous lesion studies is that the vast majority of these studies have examined individuals with specific acquired reading deficits months or years post-onset. Such chronic deficits may reflect a reorganisation of functioning, and may involve the use of compensatory mechanisms or brain regions not normally used for reading. Thus, correlations between reading patterns and lesion location in the chronic phase may not be an accurate reflection of normal reading processes.
Very few previous studies have examined reading performance acutely (within one to two days post-onset). Two studies which have done so are those of Hillis et al. (2001a), and Chen, Hillis, Pawlak, and Herskovits (2008). Both studies found that reading deficits were associated with tissue dysfunction across a distributed network of occipito-temporo-parietal regions, particularly the angular and supramarginal gyri. However, while these two studies did examine association between dysfunctional brain tissue and reading deficits in a relatively large number of acute stroke patients, both have limitations. The study of Chen et al. only examined real word reading, failing to explore potentially informative nonword reading deficits, while the study of Hillis et al. utilised a ten-region Brodmann’s area analysis based on a priori assumptions regarding the functional architecture of the brain, which may have missed potentially important brain regions involved in reading outside of those examined.
The current study attempted to examine the brain regions critical for word and nonword oral reading in acute stroke, addressing the weaknesses of our previous two studies by using voxel-based analyses of areas associated with impaired reading of familiar words and nonwords. The study differed from those conducted by other groups in that it examined a large sample of patients early after stroke to identify brain regions associated with reading deficits before extensive reorganisation. In addition, tissue dysfunction associated with both word and nonword reading performance was examined with the aim of identifying any regions differentially involved which may reflect different underlying reading processes.
Method
Participants
A series of 331 right-handed patients with acute ischemic stroke were initially enrolled upon meeting the following inclusion criteria: premorbid proficiency in English, no known hearing loss or uncorrected visual impairment; no history of dementia, previous symptomatic stroke, or other neurological disease; and no haemorrhage on initial scans. Testing was attempted to be completed within 24 hours of stroke onset; however, some patients were included who were tested between 24 and 48 hours of stroke onset (usually because they were admitted close to or after 24 hours after initial symptoms). Following enrollment, patients were selected for the current study based on the following criteria: the presence of unilateral left hemisphere infarct on MRI scans (i.e., no right hemisphere or bilateral infarcts), no infarcts involving the cerebellum or brain stem, no history of previous ischemic stroke, an education level of 10th grade or higher, and full completion of the oral reading task. Additionally, all patients underwent comprehensive neurological examinations, and any patient with eye movement disorders, visual field defects, right-sided hemispatial neglect to a sufficient degree to interfere with single word reading was excluded from the study. The final group consisted of 91 patients (50 males, 41 females), with a mean age of 59.4 ± 16.1 standard deviation (SD) years, and mean education level of 13.1 ± SD 2.2 years.
Oral Reading Task
Within 48 hours of stroke onset participants were presented with a 58 item oral reading task, involving both word and nonword reading. For the majority of patients, the task involved reading aloud 34 words and 24 nonwords (however, for a small subset of patients the task involved reading 36/22 or 35/23 words/nonwords, due to an error in the stimuli), from a mixed stimulus list presented in typed black print on white paper. Words were all morphologically-simple common nouns, of variable frequency (M = 46.05, SD = 72.48, range = 0–434), and imageability (M = 502.06, SD = 98.46, range = 317–639), and ranged in length from three to seven letters (M = 5 ± SD 1.3 letters). The nonword stimuli tended to be slightly shorter, ranging from three to five letters (M = 4.1 ± SD 0.7 letters), and were created by changing one letter of 24 words 3–5 letters in length, which were matched in frequency to the 24 3–5 letter words on the word reading list.
Although orthographic regularity was not directly manipulated or controlled for, a large proportion of the word stimuli on the current reading test had alternative possible pronunciations. These included many words with irregular or exceptional orthography to phonology (OPC) mapping (e.g., watch), and words with unique spellings (e.g., heart).
Imaging
Within 24 hours of language testing, patients underwent MR examination, including diffusion weighted imaging (DWI) with computation of apparent diffusion coefficient (ADC) maps, which reveal infarct or dense ischemia within minutes to hours of stroke onset, perfusion-weighted imaging (PWI, which reveals areas of hypoperfusion that correspond to dysfunction), Fluid Attenuated Inversion Recovery (FLAIR, which is sensitive to old infarcts), and T2*-weighted gradient-echo (which is sensitive to hemorrhage). Hypoperfusion was defined as > 4 sec delay in time to peak (TTP) arrival of contrast to the voxels within each region of interest, relative to the homologous region in the right hemisphere. In acute stroke, it is essential to identify areas of hypoperfusion as well as infarct, because both regions of dysfunctional tissue can contribute to the clinical deficits (see e.g., Hillis et al., 2001b).
Technicians blinded to the language test results identified the presence or absence of tissue dysfunction (dense ischemia or infarct defined as bright on DWI and dark on ADC maps and/or hypoperfusion on PWI, as defined above). These areas of tissue dysfunction were outlined on the MNI atlas, with regions of hypoperfusion and infarct combined to create a single lesion for each patient which encompassed all dysfunctional tissue.
Statistical Analysis
Patients were categorized into four groups: (1) No Deficit – patients who produced <10% overall error rate; (2) Nonword Deficit – patients who produced ≥10% error rate on nonwords but not words; (3) Word Deficit – patients who produced ≥10% error rate on words but not nonwords; (4) Combined Deficit – patients who produced ≥10% error rate on both words and nonwords. The cut-off of 10% was based on norms for age-matched controls for whom 10% errors was > 2 standard deviations below the mean (i.e., greater number of errors). For full details of the data analysis of patients’ reading performance and group categorisation, readers are referred to Cloutman, Newhart, Davis, Kannan, and Hillis (in press).
MRIcroN (http://www.sph.sc.edu/comd/rorden/mricron) was used to carry out a whole-brain analysis, and create a voxel-wise statistical map to show voxels where ischemia (DWI and/or PWI abnormality) was associated with word or nonword reading impairment. Two types of analyses were conducted: (1) behavioural data were treated as binary, and groups were compared (by the Liebermeister measure; Rorden, Karnath, & Bonilha, 2007; Seneta & Phipps, 2001), based on the group categorisations described above; (2) behavioural data were treated as continuous, and accuracy scores were examined across all patients (by the Brunner-Munzel test; Brunner & Munzel, 2000; Rorden, Bonilha, & Nichols, 2007), for word, nonword, and overall reading accuracy. In both analyses, lesion data were treated as a binary measure. An alpha level of 0.05 after a whole-brain False Discovery Rate (FDR) correction for multiple comparisons was used to identify significant associations in both analyses (Genovese, Lazar, & Nichols, 2002). To identify the specific brain regions associated with reading performance in these analyses, AAL brain masks were created with the WFU Pick Atlas (Maldjian, Laurienti, Burdette, & Kraft, 2003; Tzourio-Mazoyer et al., 2002). A conservative criterion of at least 10 significantly associated voxels were required in each mask region for the region to be reported as significantly associated with impairment. This was to remove brain regions where the number of significantly associated voxels were most likely too small to be strongly involved in the observed impairments.
Results
Table 1 provides a summary of patients’ behavioral performance on the oral reading task. For a detailed error analysis of the reading performance of the current patient group, see Cloutman et al. (in press), a paper that does not address the lesion sites associated with deficits. As can be seen from Table 1, only one patient produced more than 10% word errors without also producing a high rate of nonword errors. Therefore, the Word Deficit comparison was not conducted for the binary analysis.
Table 1.
Error rates as percentage of responses for each stimulus type (SD) for all patients in study, as well as specific reading groups.
| Overall | No Deficit | Word Deficit | Nonword Deficit | Combined Deficit | |
|---|---|---|---|---|---|
| n = 91 | n = 37 | n = 1 | n = 24 | n = 29 | |
| Words | 12.4 (21.9) | 0.8 (1.6) | 11.8 (0) | 3.4 (3.1) | 34.7 (27.7) |
| Nonwords | 30.3 (32.2) | 2.6 (3.6) | 8.3 (0) | 31.8 (14.5) | 65.3 (28.8) |
| Overall | 19.8 (24.6) | 1.5 (1.8) | 10.3 (0) | 15.2 (6.7) | 47.4 (25.4) |
An examination of lesion size across the three reading groups (No Deficit, Nonword Deficit, and Combined Deficit) showed a wide degree of variability in the amount of tissue damage across patients in all groups (Table 2). A Mann-Whitney U test indicated some differences in the size of lesions between groups, with the Combined Deficit group associated with significantly larger lesions than both the No Deficit (U = 335.0, Z = −2.60, p < 0.01), and Nonword Deficit (U = 220.0, Z = −2.29, p < 0.05) groups. In contrast, there was no significant difference in lesion size between the No Deficit and Nonword Deficit groups (U = 379.0, Z = −.96, n.s.).
Table 2.
Lesion size (in number of voxels) for specific reading groups.
| No Deficit | Word Deficit | Nonword Deficit | Combined Deficit | All | |
|---|---|---|---|---|---|
| n = 37 | n = 1 | n = 24 | n = 29 | n = 91 | |
| Mean | 27577 | 18582 | 13187 | 33508 | 25573 |
| SD | 63182 | - | 17669 | 32930 | 45578 |
| Median | 2269 | - | 3335 | 26174 | 6202 |
| Range | 20 – 245699 | - | 195 – 70361 | 168 – 127017 | 20 – 245699 |
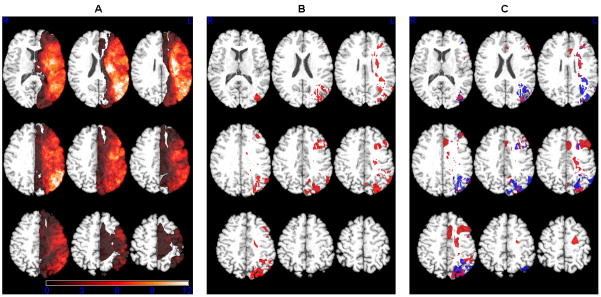
Figure 1 displays: a) a lesion overlap map showing the distribution of patient lesions; and for the continuous group analyses, the voxels of the MNI atlas where b) tissue dysfunction was associated with overall reading performance, and c) tissue dysfunction was associated with word and nonword reading. The specific brain regions significantly associated with reading deficit, and the number of voxels associated in each region, are presented in Table 3.
Figure 1.
A) Distribution map of lesion location for all patients in the current study. Voxels of the MNI atlas where tissue dysfunction (hypoperfusion and/or dense ischemia or infarct, as defined above) was associated in the continuous analysis with B) overall oral reading impairment (bright red = p < 0.01 FDR, dark red = p < 0.05 FDR), and C) word (red) and nonword (blue) reading impairment, and regions common to both (purple). Images are presented in radiological convention (left hemisphere on right).
Table 3.
Brain regions of the AAL atlas where voxels were associated with reading deficit for binary and continuous analyses.
| Binary Analysis | Continuous Analysis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No Deficit vs Any Deficita | No Deficit vs Nonword Deficit | No Deficit vs Combined Deficit | Overall | Nonword | Word | |||||||
| Sig | Vox | Sig | Vox | Sig | Vox | Sig | Vox | Sig | Vox | Sig | Vox | |
| Precentral Gyrus | * | 103 | - | - | * | 81 | ** | 142 | ** | 114 | ** | 1001 |
| Postcentral Gyrus | * | 25 | - | - | * | 24 | * | 26 | ** | 25 | * | 34 |
| Rolandic Operculum | - | - | - | - | * | 62 | - | - | - | - | * | 18 |
| Supplementary Motor Area | - | - | - | - | - | - | - | - | - | - | * | 357 |
| Superior Frontal Gyrus | - | - | - | - | * | 16 | * | 19 | - | - | * | 479 |
| Middle Frontal Gyrus | * | 1742 | * | 148 | ** | 1195 | ** | 1736 | ** | 995 | ** | 3233 |
| Inferior Frontal Gyrus – Operculum | * | 74 | - | - | * | 91 | * | 25 | * | 11 | ** | 68 |
| Inferior Frontal Gyrus – Triangular | ** | 1247 | * | 20 | ** | 382 | ** | 139 | ** | 128 | ** | 426 |
| Middle Temporal Gyrus | * | 259 | - | - | ** | 1620 | ** | 527 | ** | 645 | ** | 380 |
| Superior Parietal Lobe | * | 80 | - | - | * | 80 | ** | 104 | ** | 203 | ** | 37 |
| Supramarginal Gyrus | * | 148 | - | - | * | 105 | ** | 109 | ** | 133 | * | 48 |
| Angular Gyrus | ** | 1054 | ** | 127 | ** | 2113 | ** | 1283 | ** | 2128 | ** | 197 |
| Superior Occipital Gyrus | * | 153 | - | - | ** | 347 | ** | 840 | * | 870 | ** | 468 |
| Middle Occipital Gyrus | ** | 2079 | ** | 47 | ** | 5264 | ** | 6183 | ** | 6610 | ** | 3386 |
| Inferior Occipital Gyrus | ** | 45 | - | - | * | 100 | ** | 99 | * | 99 | ** | 131 |
| Precuneus | - | - | - | - | - | - | ** | 64 | ** | 64 | ** | 219 |
| Cuneus | - | - | - | - | - | - | ** | 56 | * | 56 | - | - |
| Anterior Cingulum | - | - | - | - | - | - | - | - | - | - | * | 646 |
| Middle Cingulum | - | - | - | - | - | - | - | - | - | - | * | 1269 |
| Posterior Cingulum | - | - | - | - | - | - | - | - | - | - | * | 107 |
| Insula | - | - | - | - | * | 33 | - | - | - | - | - | - |
| Thalamus | - | - | - | - | - | - | - | - | * | 14 | - | - |
Sig = significance level
p < 0.05
p < 0.01.
Vox = number of significant voxels in region.
‘Any Deficit’ included all patients with a >10% overall reading deficit, and included Word Deficit, Nonword Deficit, and Combined Deficit reading groups.
Both the binary and continuous analyses revealed a large network of brain regions important for reading. Specifically, the most strongly associated voxels with overall reading performance in both the binary and continuous analyses (p < 0.01) were found in the triangular part of the inferior frontal gyrus, the angular gyrus, and the middle occipital and inferior occipital gyri. Other regions also consistently associated with overall oral reading performance included the pre- and post-central gyri, the middle frontal gyrus, the opercular part of the inferior frontal gyrus, the middle temporal gyrus, superior parietal lobe, supramarginal gyrus, and the superior occipital gyrus. Examination of the number of voxels that were significantly associated in each brain region indicated that in the both the binary and continuous analyses, the greatest volume of significant voxels were found in the middle frontal, angular, and middle occipital gyri. The binary analysis also identified a large number of voxels in the triangular part of the inferior frontal gyrus.
Looking at specific word and nonword performance, as can be seen from Table 3, word reading performance in the continuous analysis was associated with those brain regions found for general reading deficits, with the most strongly associated voxels spanning a widely distributed network involving the precentral gyrus, middle frontal gyrus, inferior frontal gyrus (triangular and opercular parts), middle temporal gyrus, superior parietal lobe, angular gyrus, the superior, middle, and inferior occipital gyri, and the precuneus. Examination of the number of voxels that were significantly associated with word deficits in the continuous analysis indicated that the greatest volume of significant voxels were found in the precentral, middle frontal, and middle occipital gyri, and the middle cingulum.
For nonword reading, voxels identified in both the binary and continuous analyses included the middle frontal gyrus, the inferior frontal gyrus (triangular part), the angular gyrus, and the middle occipital gyrus. The continuous analysis indentified additional regions, most strongly in the pre- and post-central gyri, the middle temporal gyrus, the superior parietal lobe, the supramarginal gyrus, and the precuneus. Finally, voxels in the binary analysis associated with a combined word and nonword reading deficit were most strongly associated with voxels in the middle frontal gyrus, triangular part of the inferior frontal gyrus, middle temporal gyrus, angular gyrus, and the superior and middle occipital gyri. Examination of the number of voxels that were significantly associated with nonword deficits in the continuous analysis indicated that the greatest volume of significant voxels were found in the middle frontal, angular, and middle occipital gyri.
Regions that were associated with word but not nonword reading (as evidenced by significant associations in both the binary Combined Deficit and continuous Word analyses, but not the binary/continuous Nonword analyses) included the rolandic operculum and the superior frontal gyrus. Regions identified with nonword deficits but not word deficits were the cuneus and thalamus, which were only found in the continuous analysis. A comparison of the number of voxels significantly associated with word or nonword deficits in the continuous analysis indicated that word deficits were disproportionately associated with dysfunctional frontal regions, notably the precentral gyrus, supplementary motor area, superior, middle, and inferior (triangular) frontal gyri, and the cingulum (particularly the anterior and middle parts). In contrast, nonword deficits were disproportionately associated with more posterior brain regions, especially parietal regions (particularly the angular gyrus), as well as the middle temporal gyrus, and superior and middle occipital gyri.
As can be seen in Figure 1, in addition to the widely distributed cortical regions identified, damage to substantial regions of white matter were also associated with word and nonword reading impairment. Specifically, regions involving the superior longitudinal and superior fronto-occipital fasciculi appeared to be particularly implicated in both word and nonword reading.
Discussion
The current study identified a network of brain regions where acute tissue dysfunction is associated with impaired performance of oral word and nonword reading. The results indicate that oral reading crucially depends on a widely distributed network including occipital, posterior temporal (particularly the middle temporal gyrus), parietal (particularly the angular gyrus), and frontal (middle and inferior) left hemisphere brain regions. These cortical areas are consistent with those found across numerous functional imaging studies of reading with non-impaired adult populations (Jobard et al., 2003; Joseph et al., 2001), as well as the lesion locations found in chronic (Lambon Ralph & Graham, 2000; Price & Mechelli, 2005), and acute (Hillis et al., 2001; Chen et al., 2008) acquired dyslexias. Thus, the current study provides converging evidence for the importance of these brain regions in oral reading, and substantiates the essential nature of these cortical areas previously identified in functional neuroimaging studies of non-impaired adults.
Oral reading is a complex learned cognitive process which has been proposed to involve a number of key processes including visual and orthographic analysis, grapheme-phoneme integration, phonological recoding, and articulation (see e.g., Coltheart et al., 2001; Harm & Seidenberg, 2004; Plaut, 1997). Previous studies have suggested that visual feature analysis and early orthographic processing are associated with occipital and occipitotemporal regions; lexical-semantic processing with the inferior frontal gyrus (particularly the triangular part), posterior middle temporal gyrus, and the angular gyrus; and phonological processing with the posterior superior temporal gyrus, supramarginal gyrus, and the opercular part of the inferior frontal gyrus (Binder et al., 2003; Jobard et al., 2003; Mechelli et al., 2005; Salmelin & Kujala, 2006). An examination of the brain regions found to be associated with word and nonword reading in the current study appears to support a number of these previous associations. However, there are some interesting points worthy of discussion.
Previous studies have suggested that visual feature analysis and early orthographic processing are associated with occipital and occipitotemporal brain regions, with particular involvement of the lateral mid-portion of the fusiform gyrus, a region which has come to be referred to by many researchers as the ‘visual word form area’ (VWFA; Cohen et al., 2000; 2002; Vigneau, Jobard, Mazoyer, & Tzourio-Mazoyer, 2005; but see Price & Devlin, 2003, for a discussion of the debate regarding its precise role in reading). However, while the current study found substantial regions of the occipital lobe (particularly involving the middle occipital gyrus), which were associated with both word and nonword reading deficits, in contrast to previous functional imaging and lesion studies, no significant association was found between impaired oral reading performance and damage to the VWFA. An examination of the distribution of patient lesions revealed that only five of the ninety-one patients in the current study had a lesion which involved the region of the brain commonly identified as the VWFA (as defined by Vigneau et al., 2005). This reiterates an important limitation of the voxel-based lesion symptom mapping methodology in identifying brain regions important to the cognitive process under examination – while the methodology can help to elucidate the network of brain regions critically involved in the successful performance of the task, it may not necessarily identify all such regions, and is limited by the brain structures damaged in the patient sample examined.
Another brain area which has often been implicated in functional imaging studies of oral reading is the posterior temporal region, particularly the superior temporal gyrus (Fiez & Petersen, 1998; Jobard et al., 2003). However, the current study found that reading impairment was exclusively associated with areas of the middle temporal gyrus. Previous studies have often implicated the superior temporal gyrus in auditory phonological processing, and it is possible that the involvement of this region in oral reading may be more associated with auditory feedback from the reader’s vocalization of the word/nonword stimuli – a process which may be beneficial, but not essential, to task success (Fiez & Petersen, 1998). Furthermore, in relation to the middle temporal gyrus, it has also been suggested that in linguistic tasks such as reading and naming, this area may be specifically involved in lexical-semantic, rather than phonological, processing (Fiez & Petersen, 1998; Indefrey & Levelt, 2004). Consistent with this functional interpretation, the binary analyses conducted in the current study found that the middle temporal gyrus was only associated with a reading deficit which involved words as well as nonwords, and was not associated with a nonword-only reading deficit.
Oral reading involves not only the processes associated with reading but also those associated with overt speech production, including phonological processing, articulatory planning, and motor execution. Recent neuroimaging studies have identified a network of brain regions associated with overt speech production including the pars opercularis of the inferior frontal gyrus (involved in the final stages of phonological encoding and initiation of articulatory planning), the insula, basal ganglia, and the primary motor and premotor cortices (Eickhoff, Heim, Zilles, & Amunts, 2009). Several of these regions were found to be associated with oral reading deficits in the current study, specifically, the pars opercularis, insula, and precentral gyrus (primary motor cortex), further implicating these regions with the articulatory processes associated with oral word production which occur subsequent to word retrieval. The association of these regions with articulatory processes is further exemplified by a comparison of the brain regions identified in the binary analyses for the Nonword Deficit and Combined Deficit groups. Any deficits associated with articulatory planning/execution would be anticipated to affect both word and nonword production and so should be associated with a general (combined) reading deficit, rather than one restricted to only nonword oral reading, as was observed in the current study.
As noted above, the current study identified a widely distributed network of brain regions involved in the successful oral production of written words and nonwords. Accurate functioning of such a distributed functional network relies not only on the cortical regions involved, but also the connectivity between these regions, and the current study found substantial areas of white matter where damage was associated with impaired performance for both word and nonword reading (Figure 1). Previous studies which have examined the white matter fiber pathways associated with reading ability in developmentally normal children and those with dyslexia, have found a relationship between reading performance and white matter tracts connecting temporo-parietal and frontal brain areas, particularly the superior longitudinal and fronto-occipital fasciculi (see Ben-Shachar, Dougherty, & Wandell, 2007, for a review). The observation of the relationship between white matter damage and reading impairment found in the current study further extends these findings to an adult population, and emphasizes the importance of the integrity of neural fibre pathways in the successful functioning of the reading network.
Previous studies examining the processes and brain regions involved in oral reading have attempted to dissociate the different functional anatomical relationships involved based on different activation patterns found between word and nonword reading. One important limitation of the current study in the examination of these component processes was the inability to compare lesions which produced relatively selective word and nonword deficits due to the scarcity of patients who demonstrated word reading deficits in the absence of a nonword reading impairment. However, despite this limitation, there were some interesting findings regarding the brain regions associated with words and nonwords. In the current study, while word and nonword reading deficits were found to be associated with similar brain areas in general, there were differences in relation to the dominance of the different areas implicated. Brain regions associated with impaired word reading were somewhat more frontally distributed, and those associated with impaired nonword reading were distributed more posteriorly. The observation that word reading in particular was associated with larger frontal brain areas is of interest as previous neuroimaging studies have generally implicated frontal regions in phonological processing for output, although the picture is complex, and there are some areas (notably the triangular part of the inferior frontal gyrus), which have been found to be more strongly associated with word reading via lexical semantics (Jobard et al., 2003; Sandak, Mencl, Frost, & Pugh, 2004). Of interest to the current discussion are the findings of a previous behavioral error analysis which indicated that the word and nonword reading errors produced by the patients in the current study were predominantly phonological in nature, suggesting that the breakdown is indeed with some of the phonological processes involved in the oral reading of words (Cloutman et al., in press).
One possible account of this frontal dominance for the oral reading of words compared to nonwords could be due to an increased involvement of cognitive control mechanisms in the reading of words, for which both lexical and sublexical processes may produce an output for reading. Previous studies of word and nonword reading have shown that the relative contribution of lexical and sublexical reading processes may be under cognitive control, with the the dominance of either of these processes in determining the final output dependent on contextual or task requirements (Decker, Simpson, Yates, & Locker, 2003; Reynolds & Besner, 2005). This cognitive control of reading output would be particulary important in resolving competition between the two reading processes when the output responses differed (as in the case of irregular words). Thus, the greater frontal distribution associated with word reading in the current study may be associated with an increased demand on the cognitive control mechanisms involved in determining the dominance of lexical and sublexical reading outputs in the final response, and the resolution of response conflict when the two outputs differ. In support of this idea, two brain regions which have been argued to be crucially involved in cognitive control processes are the dorsolateral prefrontal cortex (DLPFC, predominantly involving the middle frontal gyrus), and the anterior cingulum (Banich, 2009; Milham, Banich, & Barad, 2003), and both of these regions were found to be more greatly associated (by statistical significance and/or number of voxels) in word reading compared to nonword reading in the current study. Importantly, the current lesion analysis found that the cingulum was exclusively associated with word reading (in the continuous analysis), and researchers have argued that this brain region (specifically the anterior portion) is strongly associated with the detection of response conflict when two (or more) competing responses are simultaneously activated, with this conflict subsequently resolved by the DLPFC (Carter & van Veen, 2007).
It could be suggested that the failure to observe patients with selective word-only reading deficits raises a question regarding the ability of the current stimuli to dissociate lexical-semantic aspects of oral reading from sublexical processes. The nonwords in the current study were created by substituting a single letter of a real word to create a phonologically legal pseudoword. There is some evidence to suggest that the ‘wordness’ of the nonword stimuli used in reading tasks can affect the processing of such stimuli. For example, studies have observed different patterns of activation in the mid-fusiform region (the VWFA) which varies as a function of the visual familiarity of the stimulus, with a greater resemblance of nowords to real words producing greater similarity in activation patterns to those produced by word stimuli (Proverbio & Adorni, 2008; Vinckier et al., 2007). Thus, it is possible that the large number of Combined Deficit patients in the current study, and similarity in brain regions observed for word and nonword stimuli, was associated with the high degree of ‘wordness’ of the nonword stimuli used in the oral reading task. However, while there appears to be increasing evidence to suggest that the degree of similarity to words can affect the visual processing of nonword stimuli, the extent to which this similarity may affect the processes involved in subsequent orthography-phonology conversion (and the corresponding brain regions involved), still remains unclear. Studies which have manipulated and compared the effect of the similarity of nonwords to real words have identified several brain regions which appear to be associated with reading processes occurring post visual identification, such as the supplementary motor area, superior temporal sulcus, and Broca’s area (Vinckier et al., 2007). However, within such studies, the ‘wordness’ of a nonword is often confounded with pronounceablility, and there is evidence to indicate that phonological legality may be an important variable in such brain regions, particularly in temporo-parietal areas (Proverbio et al., 2008). In addition, it should be noted that although the current study was unable to identify a substantial number of patients with a selective word reading deficit, the stimuli used was able to dissociate those patients who demonstrated impaired nonword reading in the face of preserved word reading, indicating a degree of sensitivity in the identification of word and nonword reading impairment.
In comparing word and nonword reading, one of the key aims is to attempt to dissociate proposed lexical-semantic reading processes from phonological processes. However, as words with regular spelling-sound correspondences can be read via both lexical and sublexical reading processes, in addition to a comparison of words versus nonwords, many researchers have also manipulated the orthographic regularity and frequency of the word stimuli used. Due to the limitations associated with testing patients within the first 24 hours following stroke onset, the current study was restricted to the comparison of words versus nonwords only, using word stimuli which were all nouns with simple morphography, and which were limited to a range of phoneme/grapheme lengths (and matched to the nonwords on the same), within a restricted range of frequencies. As only words and nonwords were being contrasted in this study, frequency and regularity were not a focus, as they are irrelevant for nonwords. It should be noted, however, that a large proportion of the word stimuli on the reading test used had alternative possible pronunciations, and included many words with irregular orthography-to-phonology (OPC) mappings or unique spellings. As such, it is important to note that it would not be possible to achieve normal performance in reading words on this test by applying the most common OPC mappings to read the stimuli. Nevertheless, one limitation of the current study was the fact that the regular, irregular, and exception words used were not matched for length and frequency. Therefore, the relationship between regions of dysfunction and impairment in reading regular versus irregular or exception words was unable to be analysed, since orthographic regularity may have been confounded by length and/or frequency.
Conclusion
Oral reading is a complex skill involving the interaction of orthographic, phonological, and semantic processes. The identification of brain regions associated with reading is important as it can help to inform our understanding of the processes involved, and may help to uncover the mechanisms underlying reading deficits following brain damage. However, despite accumulating data, the brain regions underlying the processes critical for reading remain poorly understood. The current study attempted to elucidate the brain regions critical for oral reading by examining lesion-symptom correlates in a large number of patients in the acute phase of stroke. Reading performance in the acute phase is of interest as it allows the identification of brain regions essential for normal reading without the potential for cognitive or neuroanatomical reorganization of functioning. A widely distributed network of brain regions were found to be associated with word and nonword oral reading performance, providing complementary evidence for the importance of several key cortical regions in the left inferior frontal, inferior parietal, posterior temporal, occipito-temporal and occipital regions, which are also engaged in reading in functional imaging studies.
Acknowledgments
The research reported in this paper was supported by NIH (NIDCD), through RO1 DC 05375. We gratefully acknowledge this support and the participation of the patients.
Dr. Lauren Cloutman is now at the Neuroscience and Aphasia Research Unit (NARU), School of Psychological Sciences, University of Manchester, UK.
Footnotes
Disclosure
The authors have no relationships to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banich MT. Executive function: The search for an integrated account. Current Directions in Psychological Science. 2009;18:89–94. [Google Scholar]
- Ben-Shachar M, Dougherty RF, Wandell BA. White matter pathways in reading. Current Opinion in Neurobiology. 2007;17:258–270. doi: 10.1016/j.conb.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Binder JR, McKiernan KA, Parsons ME, Westbury CF, Possing ET, Kaufman JN, Buchanan L. Neural correlates of lexical access during visual word recognition. Journal of Cognitive Neuroscience. 2003;15:372–393. doi: 10.1162/089892903321593108. [DOI] [PubMed] [Google Scholar]
- Brunner E, Munzel U. The nonparametric Behrens-Fisher problem: Asymptotic theory and a small-sample approximation. Biometrical Journal. 2000;42:17–25. [Google Scholar]
- Bub D. Alexia and related reading disorders. Neurologic Clinics of North America. 2003;21:549–568. doi: 10.1016/s0733-8619(02)00099-3. [DOI] [PubMed] [Google Scholar]
- Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: An update of theory and data. Cognitive, Affective, and Behavioural Neuroscience. 2007;7:367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- Chen R, Hillis AE, Pawlak M, Herskovits EH. Voxelwise Bayesian lesion deficit analysis. Neuro Image. 2008;40:1633–1642. doi: 10.1016/j.neuroimage.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutman LL, Newhart M, Davis CL, Kannan VC, Hillis AE. Patterns of reading performance in acute stroke: A descriptive analysis. Behavioural Neurology. doi: 10.3233/BEN-2009-0258. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehericy S, Dehaene-Lambertz G, Henaff MA, Michel F. The visual word form area: Spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123:291–307. doi: 10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- Cohen L, Lehericy S, Chochon F, Lemer C, Rivaud S, Dehaene S. Language-specific tuning of visual cortex? Functional properties of the visual word form area. Brain. 2002;125:1054–1069. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- Coltheart M, Rastle K, Perry C, Ziegler J, Langdon R. DRC: A dual route cascaded model of visual word recognition and reading aloud. Psychological Review. 2001;108:204–256. doi: 10.1037/0033-295x.108.1.204. [DOI] [PubMed] [Google Scholar]
- Davis C, Hillis A, Bergey G, Ritzl E. Who needs Broca’s area? Comparisons from lesion and fMRI methods. Brain and Language. 2007;103:14–15. [Google Scholar]
- Decker G, Simpson GB, Yates M, Locker L. Flexible use of lexical and sublexical information in word recognition. Journal of Research in Reading. 2003;26:280–286. [Google Scholar]
- Eickhoff SB, Heim S, Zilles K, Amunts K. A systems perspective on the effective connectivity of overt speech production. Philosophical Transactions of the Royal Society A. 2009;376:2399–2421. doi: 10.1098/rsta.2008.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiez JA, Petersen SE. Neuroimaging studies of word reading. Proceedings of the National Academy of Sciences USA. 1998;95:914–921. doi: 10.1073/pnas.95.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuro Image. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Hamilton AC, Coslett HB. Role of inflectional regularity and semantic transparency in reading morphologically complex words: Evidence from acquired dyslexias. Neuro Case. 2008;14:347–368. doi: 10.1080/13554790802368679. [DOI] [PubMed] [Google Scholar]
- Harm MW, Seidenberg MS. Computing the meaning of words in reading: Cooperative division of labour between visual and phonological processes. Psychological Review. 2004;111:662–720. doi: 10.1037/0033-295X.111.3.662. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Kane A, Barker P, Beauchamp N, Gordon B, Wityk R. Neural substrates of the cognitive processes underlying reading: Evidence from magnetic resonance perfusion imaging in hyperacute stroke. Aphasiology. 2001a;15:919–931. [Google Scholar]
- Hillis AE, Wityk RJ, Tuffiash E, Beauchamp NJ, Jacobs MA, Barker PB, Selnes OA. Hypoperfusion of Wernicke’s area predicts severity of semantic deficit in acute stroke. Annals of Neurology. 2001b;50:561–566. doi: 10.1002/ana.1265. [DOI] [PubMed] [Google Scholar]
- Indefrey P, Levelt WJM. The spatial and temporal signatures of word production components. Cognition. 2004;92:101–144. doi: 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- Jobard G, Crivello F, Tzourio-Mazoyer N. Evaluation of the dual route theory of reading: A meta-analysis of 35 neuroimaging studies. Neuro Image. 2003;20:693–712. doi: 10.1016/S1053-8119(03)00343-4. [DOI] [PubMed] [Google Scholar]
- Joseph J, Noble K, Eden G. The neurobiological basis of reading. Journal of Learning Disabilities. 2001;34:566–579. doi: 10.1177/002221940103400609. [DOI] [PubMed] [Google Scholar]
- Laine M, Niemi P, Niemi J, Koivuselka-Sallinen P. Semantic errors in deep dyslexia. Brain and Language. 1990;38:207–214. doi: 10.1016/0093-934x(90)90111-s. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Graham NL. Acquired phonological and deep dyslexia. Neuro Case. 2000;6:141–178. [Google Scholar]
- Leff AP, Crewes H, Plant GT, Scott SK, Kennard C, Wise RJS. The functional anatomy of single-word reading in patients with hemianopic and pure alexia. Brain. 2001;124:510–521. doi: 10.1093/brain/124.3.510. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JB, Kraft RA. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuro Image. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Crinion JT, Long S, Friston KJ, Lambon Ralph MA, Patterson K, McClelland JL, Price CJ. Dissociating reading processes on the basis of neuronal interactions. Journal of Cognitive Neuroscience. 2005;17:1753–1765. doi: 10.1162/089892905774589190. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Barad V. Competition for priority in processing increases prefrontal cortex’s involvement in top-down control: An event-related fMRI study of the stroop task. Cognitive Brain Research. 2003;17:212–222. doi: 10.1016/s0926-6410(03)00108-3. [DOI] [PubMed] [Google Scholar]
- Plaut DC. Structure and function in the lexical system: Insights from distributed models of word reading and lexical decision. Language and Cognitive Processes. 1997;12:765–805. [Google Scholar]
- Plaut DC, McClelland JL, Seidenberg MS, Patterson K. Understanding normal and impaired word reading: Computational principles in quasi-regular domains. Psychological Review. 1996;103:56–115. doi: 10.1037/0033-295x.103.1.56. [DOI] [PubMed] [Google Scholar]
- Price CJ, Devlin JT. The myth of the visual word form area. Neuro Image. 2003;19:473–481. doi: 10.1016/s1053-8119(03)00084-3. [DOI] [PubMed] [Google Scholar]
- Price CJ, Mechelli A. Reading and reading disturbance. Current Opinion in Neurobiology. 2005;15:231–238. doi: 10.1016/j.conb.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Proverbio AM, Adorni R. Orthographic familarity, phonological legality and number of orthographic neighbours affect the onset of ERP lexical effects. Behavioural and Brain Functions. 2008;4:27. doi: 10.1186/1744-9081-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, Shaywitz SE, Shaywitz BA. Neurobiological studies of reading and reading disability. Journal of Communication Disorders. 2001;34:479–492. doi: 10.1016/s0021-9924(01)00060-0. [DOI] [PubMed] [Google Scholar]
- Rapcsak SZ, Beeson PM, Henry ML, Leyden A, Kim E, Rising K, Andersen S, Cho H. Phonological dyslexia and dysgraphia: Cognitive mechanisms and neural substrates. Cortex. 2009;45:575–591. doi: 10.1016/j.cortex.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds M, Besner D. Contextual control over lexical and sublexical routines when reading English aloud. Psychonomic Bulletin and Review. 2005;12:113–118. doi: 10.3758/bf03196355. [DOI] [PubMed] [Google Scholar]
- Rorden C, Bonilha L, Nichols TE. Rank-order versus mean based statistics for neuroimaging. Neuro Image. 2007;35:1531–1537. doi: 10.1016/j.neuroimage.2006.12.043. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath HO, Bonilha L. Improving lesion-symptom mapping. Journal of Cognitive Neuroscience. 2007;19:1081–1088. doi: 10.1162/jocn.2007.19.7.1081. [DOI] [PubMed] [Google Scholar]
- Salmelin R, Kujala J. Neural representation of language: activation versus long-range connectivity. Trends in Cognitive Sciences. 2006;10:519–525. doi: 10.1016/j.tics.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Sandak R, Mencl WE, Frost SJ, Pugh KR. The neurobiological basis of skilled and impaired reading: Recent findings and new directions. Scientific Studies of Reading. 2004;8:273–292. [Google Scholar]
- Schlagger BL, McCandliss BD. Development of neural systems for reading. Annual Review of Neuroscience. 2007;30:475–503. doi: 10.1146/annurev.neuro.28.061604.135645. [DOI] [PubMed] [Google Scholar]
- Seidenberg MS, McClelland JL. A distributed, developmental model of word recognition and naming. Psychological Review. 1989;96:523–568. doi: 10.1037/0033-295x.96.4.523. [DOI] [PubMed] [Google Scholar]
- Seneta E, Phipps MC. On the comparison of two observed frequencies. Biometrical Journal. 2001;43:23–43. [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: Method and validation. Neuro Image. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuro Image. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Jobard G, Mazoyer B, Tzourio-Mazoyer N. Word and non-word reading: What role for the visual word form area? Neuro Image. 2005;27:694–705. doi: 10.1016/j.neuroimage.2005.04.038. [DOI] [PubMed] [Google Scholar]
- Vinckier F, Dehaene S, Jobert A, Dubus JP, Sigman M, Cohen L. Hierarchical coding of letter strings in the ventral stream: Dissecting the inner organisation of the visual word-form system. Neuron. 2007;55:143–156. doi: 10.1016/j.neuron.2007.05.031. [DOI] [PubMed] [Google Scholar]