Abstract
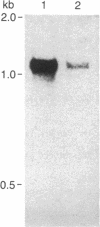
Two sets of synthetic oligonucleotides coding for amino acids in the amino- and carboxyl-terminal portions of wheat germ agglutinin were synthesized and used as hybridization probes to screen cDNA libraries derived from developing embryos of tetraploid wheat. The nucleotide sequence for a cDNA clone recovered from the cDNA library was determined by dideoxynucleotide chain-termination sequencing in vector M13. The amino acid sequence deduced from the DNA sequence indicated that this cDNA clone (pNVR1) encodes isolectin 3 of wheat germ agglutinin. Comparison of the deduced amino acid sequence of clone pNVR1 with published sequences indicates isolectin 3 differs from isolectins 1 and 2 by 10 and 8 amino acid changes, respectively. In addition, the protein encoded by pNVR1 extends 15 amino acids beyond the carboxyl terminus of the published amino acid sequence for isolectins 1 and 2 and includes a potential site for N-linked glycosylation. Utilizing the insert of pNVR1 as a hybridization probe, we have demonstrated that the expression of genes for wheat germ agglutinin is modulated by exogenous abscisic acid. Striking homology is observed between wheat germ agglutinin and chitinase, both of which are proteins that bind chitin.
Keywords: lectin, wheat
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A. K., Neuberger A., Sharon N. The purification, composition and specificity of wheat-germ agglutinin. Biochem J. 1973 Jan;131(1):155–162. doi: 10.1042/bj1310155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broglie K. E., Gaynor J. J., Broglie R. M. Ethylene-regulated gene expression: molecular cloning of the genes encoding an endochitinase from Phaseolus vulgaris. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6820–6824. doi: 10.1073/pnas.83.18.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C., Tamaki S., Dunsmuir P., Favreau M., Katayama C., Dooner H., Bedbrook J. mRNA transcripts of several plant genes are polyadenylated at multiple sites in vivo. Nucleic Acids Res. 1986 Mar 11;14(5):2229–2240. doi: 10.1093/nar/14.5.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson M. L., Rödin J., Lenman M., Glimelius K., Josefsson L. G., Rask L. Structure of the rapeseed 1.7 S storage protein, napin, and its precursor. J Biol Chem. 1986 Nov 5;261(31):14576–14581. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Goldstein I. J., Hayes C. E. The lectins: carbohydrate-binding proteins of plants and animals. Adv Carbohydr Chem Biochem. 1978;35:127–340. doi: 10.1016/s0065-2318(08)60220-6. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Methods Enzymol. 1983;100:333–342. doi: 10.1016/0076-6879(83)00066-x. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lis H., Sharon N. Lectins as molecules and as tools. Annu Rev Biochem. 1986;55:35–67. doi: 10.1146/annurev.bi.55.070186.000343. [DOI] [PubMed] [Google Scholar]
- Mirelman D., Galun E., Sharon N., Lotan R. Inhibition of fungal growth by wheat germ agglutinin. Nature. 1975 Jul 31;256(5516):414–416. doi: 10.1038/256414a0. [DOI] [PubMed] [Google Scholar]
- Mishkind M. L., Palevitz B. A., Raikhel N. V., Keegstra K. Localization of wheat germ agglutinin--like lectins in various species of the gramineae. Science. 1983 Jun 17;220(4603):1290–1292. doi: 10.1126/science.220.4603.1290. [DOI] [PubMed] [Google Scholar]
- Mishkind M., Raikhel N. V., Palevitz B. A., Keegstra K. Immunocytochemical localization of wheat germ agglutinin in wheat. J Cell Biol. 1982 Mar;92(3):753–764. doi: 10.1083/jcb.92.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata Y., Burger M. M. Wheat germ agglutinin. Molecular characteristics and specificity for sugar binding. J Biol Chem. 1974 May 25;249(10):3116–3122. [PubMed] [Google Scholar]
- Pustell J., Kafatos F. C. A convenient and adaptable package of computer programs for DNA and protein sequence management, analysis and homology determination. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):643–655. doi: 10.1093/nar/12.1part2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triplett B. A., Quatrano R. S. Timing, localization, and control of wheat germ agglutinin synthesis in developing wheat embryos. Dev Biol. 1982 Jun;91(2):491–496. doi: 10.1016/0012-1606(82)90057-4. [DOI] [PubMed] [Google Scholar]
- Williamson J. D., Quatrano R. S., Cuming A. C. Em polypeptide and its messenger RNA levels are modulated by abscisic acid during embryogenesis in wheat. Eur J Biochem. 1985 Oct 15;152(2):501–507. doi: 10.1111/j.1432-1033.1985.tb09224.x. [DOI] [PubMed] [Google Scholar]
- Wright C. S., Gavilanes F., Peterson D. L. Primary structure of wheat germ agglutinin isolectin 2. Peptide order deduced from X-ray structure. Biochemistry. 1984 Jan 17;23(2):280–287. doi: 10.1021/bi00297a017. [DOI] [PubMed] [Google Scholar]
- Wright C. S., Olafsdottir S. Structural differences in the two major wheat germ agglutinin isolectins. J Biol Chem. 1986 Jun 5;261(16):7191–7195. [PubMed] [Google Scholar]
- Wright C. S. Refinement of the crystal structure of wheat germ agglutinin isolectin 2 at 1.8 A resolution. J Mol Biol. 1987 Apr 5;194(3):501–529. doi: 10.1016/0022-2836(87)90678-4. [DOI] [PubMed] [Google Scholar]
- Wright C. S. Structural comparison of the two distinct sugar binding sites in wheat germ agglutinin isolectin II. J Mol Biol. 1984 Sep 5;178(1):91–104. doi: 10.1016/0022-2836(84)90232-8. [DOI] [PubMed] [Google Scholar]