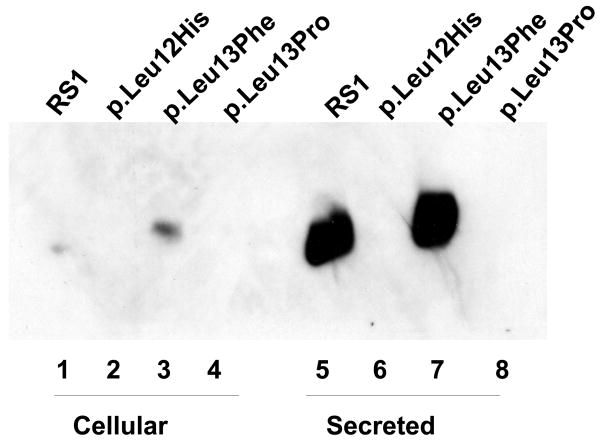
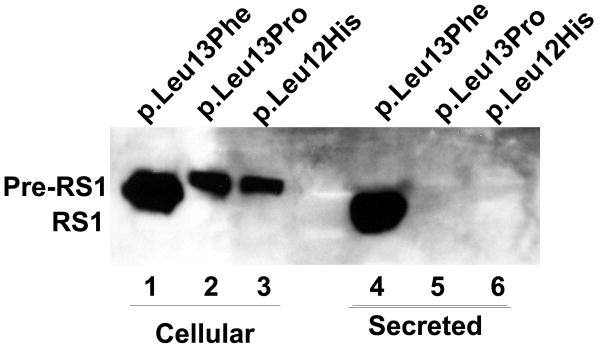
Figure 5.
Immunoblot analysis of RS1 expression in cellular and secreted fractions of COS-7 cells expressing p.Leu12His and p.Leu13Pro mutants. (A) The WT RS1 and the p.Leu13Phe mutant with intact signal sequence hydrophobic core both profusely expressed RS1, which was processed and secreted into the culture medium (lanes 5, 7) with little retained in the cellular fractions (lanes 1, 3). The p.Leu12His (lanes 2, 6) and p.Leu13Pro mutants (lanes 6, 8), with a disrupted hydrophobic core, showed virtual lack of RS1 expression. (B) Proteasomal inhibition stabilizes RS1 protein in the mutants. COS-7 cells were transfected with RS1 expression plasmids one day before MG132 (10 μM for 6h); or its vehicle dimethyl sulfoxide (DMSO) was added, and the cells were grown in serum-supplemented medium. The precursor form of RS1 (224 aa) stabilized in the presence of MG132 is seen in the cellular fractions of the signal sequence mutants (lanes 1–3). In p.Leu13Phe the precursor is processed into mature form of RS1 (201 aa) and secreted into the medium (lane 4) but not in the p.Leu12His and p.Leu13Pro mutants due to a processing defect.