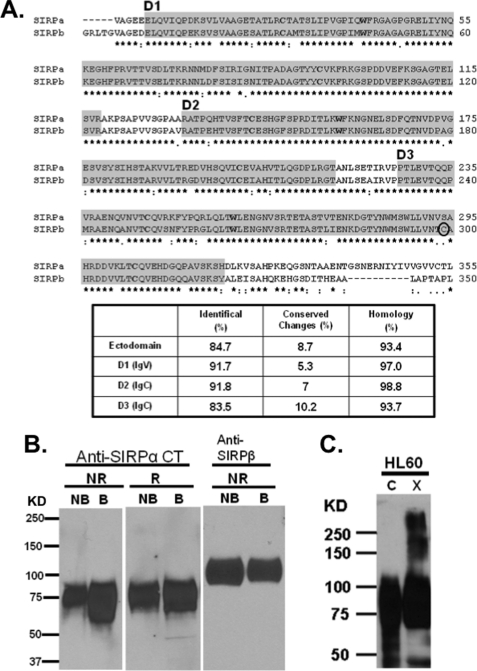
FIGURE 1.
SIRPα is highly similar to SIRPβ and dimerizes after chemical cross-linking in HL60 cells. A, alignment of SIRPα and SIRPβ ectodomains revealing identical (stars) and conserved (dots) residues. The peptide sequences that represent each of the Ig folds as defined by Protein Family databases (PFAM) and the crystallization study are enclosed in gray boxes. The cysteine and tryptophan residues conserved in Ig folds are in boldface type. SIRPβ dimerization is mediated through a disulfide linkage at cysteine residue Cys299 (circled) as reported previously (31). B, immunoblots of HL-60 cell lysates under non-reduced (NR) and reduced (R) conditions with boiling (B) or without boiling (NB) were probed using rabbit antiserum against SIRPα cytoplasmic tail (Anti-SIRPαCT) and murine monoclonal antibody specific against SIRPβ (B4B6). Anti-SIRPβ antibody was unable to detect SIRPβ under reducing conditions (data not shown). C, immunoblot analysis using a rabbit polyclonal antibody against the SIRPα cytoplasmic tail detecting noncovalently linked SIRPα dimers after treatment of HL60 cells with a cell membrane-impermeable chemical cross-linker BS3. Results are representative of one of five independent experiments.