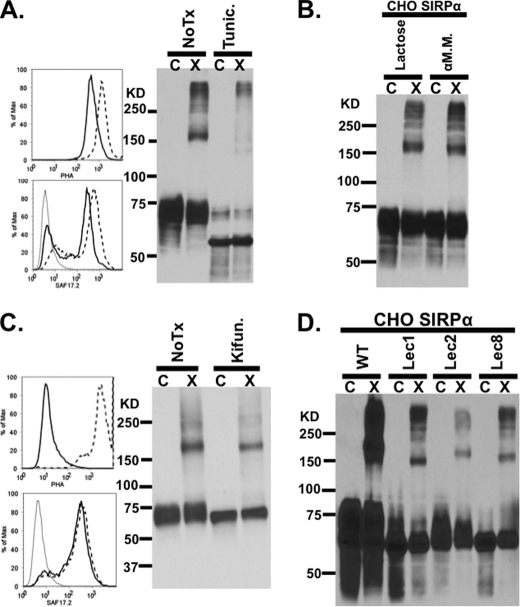
FIGURE 6.
SIRPα dimerization is disrupted after treatment with tunicamycin. A, flow cytometric analysis of tunicamycin-treated (solid line) and untreated (dashed line) CHO-SIRPα transfectants labeled with PHA-L-FITC (top left panel) to measure the degree of deglycosylation and with anti-SIRPαD1 (SAF17.2; bottom left panel) to assess the level of SIRPα expression. After cross-linking, treated cells were analyzed by immunoblots for the presence of SIRPα dimers. B, immunoblot after cross-linking, demonstrating that SIRPα on CHO cells still dimerized in the presence of 100 mm α-methyl-d-mannoside or 100 mm lactose, which were added to competitively inhibit potential dimerization by multivalent mannose or galatose binding lectins. C, flow cytometric analysis of kifunensine-treated CHO-SIRPα transfectants labeled with PHA-L-FITC (top left panel) to measure the degree of deglycosylation and with anti-SIRPαD1 (SAF17.2; bottom left panel) to assess the level of SIRPα expression. After cross-linking, treated cells were analyzed by immunoblots for the presence of SIRPα dimers. D, immunoblot after cross-linking demonstrating that SIRPα expressed in mutant CHO cells lacking enzymes necessary for terminal modification of N-glycans, such as Lec1 (mannose-rich), Lec2 (sialic acid-deficient), and Lec8 (galactose-deficient), can still dimerize. Results are representative of one of four independent experiments. C, control; X, cross-linked.