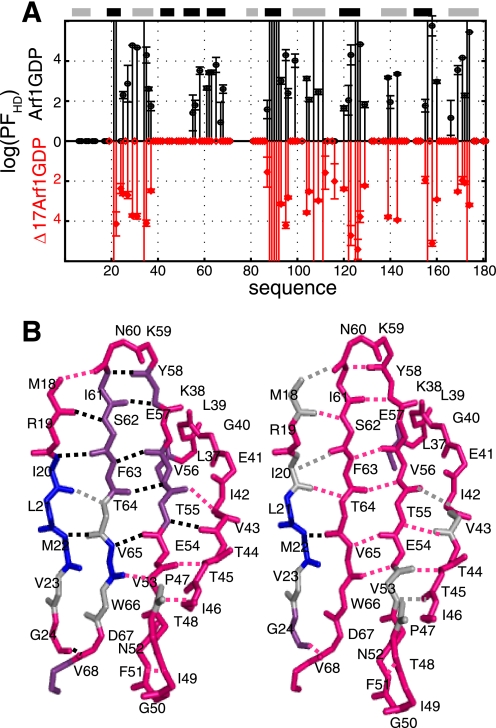
FIGURE 6.
Native-state H/D exchange experiments. A, logarithm of solvent protection factors (log(PFHD)) of the amide protons of Arf1-GDP (black) and Δ17Arf1-GDP (red) as a function of the protein sequence. Zero values correspond to residues whose amide protons are already fully exchanged in the first HSQC spectrum. Values higher than six correspond to residues that were not exchanged after 120 h. B, H/D exchange in the interswitch region and flanking β1 and switch 1 β-strand in Arf1-GDP (left) and Δ17Arf1-GDP (right). Residues whose amide protons exchange with deuterium faster than minutes, in the order of hours, and slower than a week are represented in pink, purple, and blue, respectively. Amide protons of residues that cannot be assigned are shown in gray (ND). Hydrogen bonds are highlighted in pink for those involving fast exchanged amide protons (<min), in gray for ND, and in black otherwise.