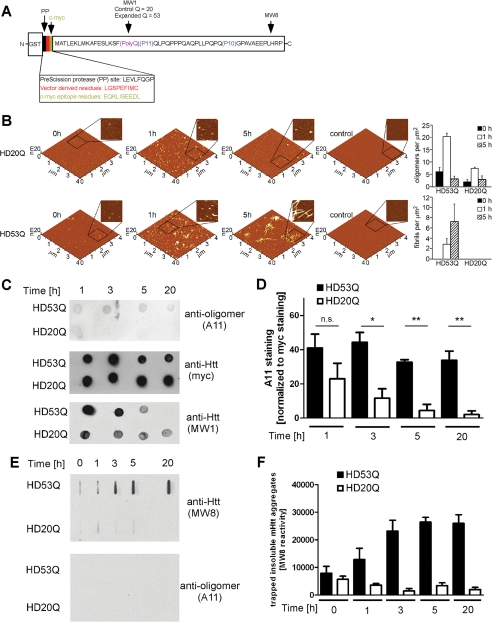
FIGURE 1.
The conformation-dependent anti-oligomer antibody A11 detects mutant htt oligomers but not monomers or fibrils. A, schematic of the GST-mutant htt exon 1 fusion protein with 53Q (HD53Q) showing a PreScission protease site between GST and the mutant htt fragment (not drawn to scale) and the locations of epitopes for MW8, MW1, and c-Myc antibodies. B, morphology of HD20Q and HD53Q aggregation reactions over time, analyzed by AFM. Representative AFM images of 6 μm aggregation reactions at 0, 1, and 5 h are shown. Zoomed in panels are 1 μm by 1 μm. Controls are denatured HD20Q and HD53Q in reaction buffer with 6 m guanidinium HCl. Analysis of oligomers and fibrils per unit area at 0, 1, and 5 h is presented. C, dot-blots of HD53Q and HD20Q after incubation with protease for various times, probed with antibodies as labeled. D, percentage of A11 reactivity relative to Myc reactivity (100%) in dot-blots of three independent experiments, quantified by densitometry using Image J. *, p < 0.05 versus HD20Q. ns, not significant at 1 h by t test. The values are the means ± S.E. E, filter trap assays of HD53Q and HD20Q after incubation with protease for various times. Insoluble HD53Q oligomers and fibrils were trapped and detected by MW8 but not by A11. F, quantification of filter trap assay of three independent experiments, quantified by densitometry using Image J. The values are the means ± S.E.