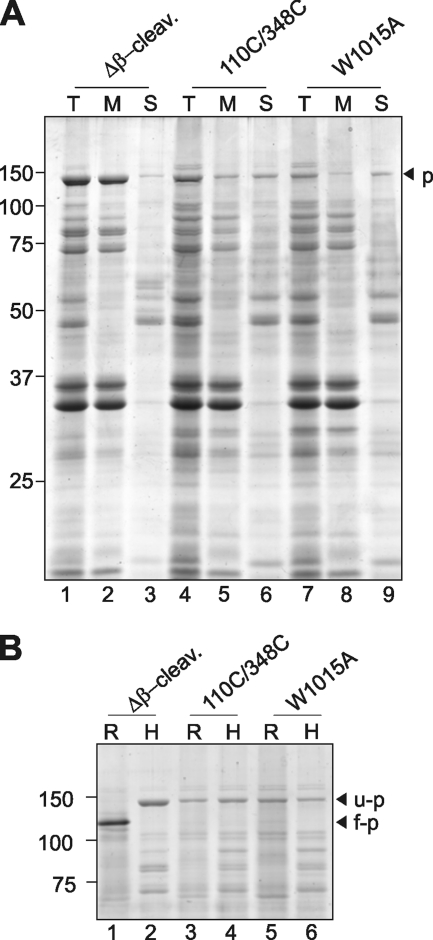
FIGURE 4.
The β-domain of HbpW1015A mutant is not fully inserted and folded in the OM. A, Coomassie Brilliant Blue-stained SDS-PAGE of urea-treated outer OM preparations from cells expressing the indicated Hbp derivatives. Fractions containing the outer membranes, as described in the legend for Fig. 3 (HSP), were treated with 4 m urea for 30 min on ice. Solubilized material (S) was separated from the integral membrane fraction (M) by centrifugation at 183,800 × g for 30 min and analyzed by SDS-PAGE and Coomassie Brilliant Blue staining. Input material was included for reference (T). Δβ-cleav., Δβ-cleavage; p, pro-form. B, Coomassie Brilliant Blue-stained PAGE of semi-native and heat-treated OM preparations from cells expressing the indicated Hbp derivatives. Fractions containing the outer membranes were either solubilized in sample buffer containing 0.2% SDS and incubated at room temperature (R) or solubilized in sample buffer containing 2% SDS at 95 °C for 10 min (H). Unfolded (u-p) and folded (f-p) forms of pro-Hbp are indicated on the right side of the panels; the identity of pro-Hbp was confirmed by immunoblotting (data not shown).